The liquid state of matter is characterized by short-ranged correlations, as opposed to the long-ranged correlations of crystals and the absence of correlations in an ideal gas. Use this tag for questions specifically concerning the thermodynamics and statistical-mechanics of liquids. For the dynamical and mechanical properties of liquids, use the "fluid-dynamics" and "fluid-statics" tags.
The liquid state of matter is intuitively perceived as that state between a gas and a solid.
This intuition can be formally expressed if we consider the kinetic energy $K$ and the potential energy $U$ of the system under examination. In a gas, the kinetic energy far exceeds the interaction energy: $K/|U| \gg 1$. In a solid, the opposite is true: $K/|U| \ll 1$. In the liquid state, the contributions of kinetic and potential energy are similar: $K/|U| \simeq 1$.
While an ideal gas is characterized by the absence of correlations between its particles and a crystalline solid is characterized by long-ranged correlations, a liquid presents short-ranged correlations, whose origin lays in the strongly repulsive forces generated at short distances by the typical intermolecular potentials.
Such repulsive forces are quantum mechanical in origin: they arise as a consequence of the Pauli exclusion principle. The typical intermolecular potential will therefore have a steep repulsive part at short distances. A well-known example is provided by the Lennard-Jones potential:
$$u(r) = 4 \epsilon \left[ \left(\frac \sigma r\right)^{12}-\left(\frac \sigma r\right)^{6} \right]$$
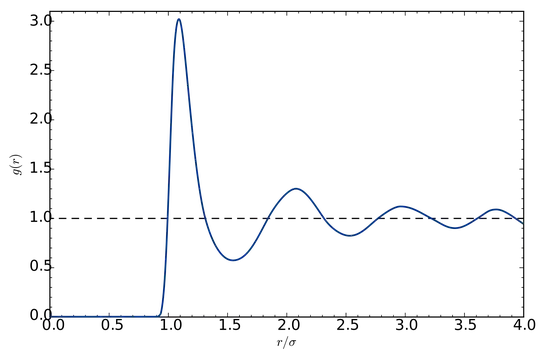
These correlations can be quantified by computing the radial distribution function $g(r)$ of the system: for an ideal gas, $g(r)=1$. For a crystalline solid, it is an infinite succession of sharp peaks. For a liquid, it presents strong oscillations at short distances, and it goes to $1$ at large distances.
Prerequisites:
- Thermodynamics
- Statistical Mechanics
Books on the physics of liquids:
The main book about simple classical liquids is Hansen-McDonald, Theory of Simple Liquids.