I think the problem here is with E&M, and it is in the assumptions implicit to the question. Here is the assumption:
the electron orbiting around the nucleus has an acceleration. Therefore it radiates and loses energy, until it would collapse with the nucleus.
This statement can be demolished in short order, considering the topic of a non-radiating condition. The definition of the condition is:
the conditions according to classical electromagnetism under which a distribution of accelerating charges will not emit electromagnetic radiation.
The statement that acceleration leads to radiation and therefore collapse must be completely abandoned. We still have room for skepticism - formally we are arguing over whether the Bohr model of an electron fits the radiating condition.
I will argue that there is not a simple answer "yes" or "no". The reason is that we could currently re-interpret it as a model where the answer is that it does not fit a radiating condition. The picture of the Bohr atom I am most familiar with looks like this:
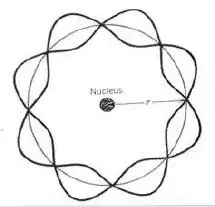
This is, an electron moving around a nucleus doing two things, a) exhibiting orbital behavior and b) fitting the orbital circumference to a mode of its de Broglie wavelength. But is the electron a particle or a wave in this interpretation? Following from that, does the electron have a defined location? If it's a defined particle, then it must radiate. But then why the requirement that the wavelength fit in the first place?
Let's turn to a classical analog. Imagine a ring of wire with no resistance and current flowing. This is an ideal electromagnet. It has a static electric field and a static magnetic field, but nothing is changing. It will sit there and maintain the current forever. (img source)
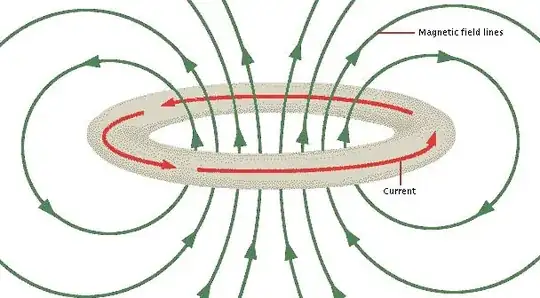
What's more, we could paint a picture of a simple electron orbital that fits this. Mentally combine the above two images. You'll probably be confused about the peaks and troughs of the wave, since they are not smooth with respect to position along the orbit. But this is an electron wave, not a EM wave. You can not distinguish the peaks and troughs in reality because the probability density is the imaginary wave function squared.
This doesn't describe all orbitals perfectly. For one, the electron in this model has a net angular momentum. But still, this gets surprisingly close to a passable model of nature. I think this is the correct stepping stone to move between the Bohr atom and real QM. The only question not sufficiently answered is why the electron arranges itself in this torus-like state, and the answer is that it doesn't because the actual dynamics are explained by QM, which not only accurately describes the emission lines (better than the orbital calculations confined to wavelength intervals), but also correctly predicts chemical bonds and the entire world around us.
What Schrödinger (really QM in general) did to advance beyond the Bohr model was to give a correct physical justification of the use of electron wavelength - leading to the wave equation. In this regard, the Bohr model was a confused mess, but it could be tweaked to be non-radiating per my above argument, which entails non-localization of the electron along the circumference of orbit. Obviously this is nonsense, because if it's non-local along that line, then why isn't it non-local along other dimensions as well? QM answered this correctly.

