I was reading that Bohr assumed electrons in orbit simply did not radiate, and my professor told me that the actual case is that electrons are clouds of probability. Even so, aren't they still moving around? I mean, if they are particles, then they should be moving around-even if you can't pinpoint them. Then, shouldn't they radiate?
1 Answers
my professor told me that the actual case is that electrons are clouds of probability.
When one is talking of elementary particles, and the electron is one of the elementary particles in the Standard Model, one has to understand quantum mechanics. The underlying substrate of Nature is quantum mechanical; classical physics emerges when the orders of magnitude are such that the heisenberg uncertainty principle is trivially fulfilled, because of the small value of h, the Planck constant.
Your professor is speaking of the quantum dynamical solutions for any situation in the microcosm. There is no orbit for an electron around the nucleus, there is an orbital. The only thing we know about the electron in the orbital is its energy and various quantum numbers of the energy level, and what the probability is if we do an experiment to find the electron at an (x.y,z) point. The probability. We cannot compute an orbit and expect the electron to be running predictably around, the way the moon goes around the earth and compute its (x,y,z) for every t . This has no meaning in the microcosm, only the probability is computable.
Even so, aren't they still moving around? I mean, if they are particles, then they should be moving around-even if you can't pinpoint them.
Their motion is not the classical motion. If an electron is hit by a photon it can fly away in a trajectory we can macroscopicaly see and compute from the energy balances of the problem. But we can only talk of how probable it is that the photon will hit the electron.
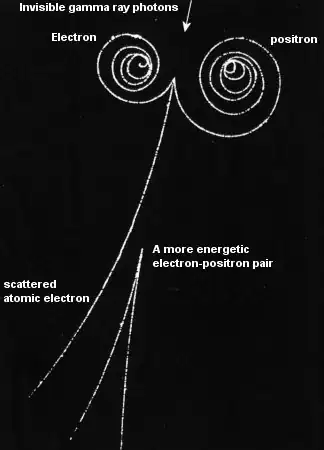
Then, shouldn't they radiate?
A bound in a potential electron does not radiate. An electron moving through matter has a probability to radiate as in the photo above, from the field of the bound electrons in the medium, and again a computable probability.
In this case the calculation of the probability of radiation depends on the energy that will radiate and the formulae have been experimentally checked. The probability of radiating small energy photons and interacting with the electrons ionizing the medium so that we can see the track has also been computed.
That is the way it works at the basic level of nature, quantum mechanically, probabilities.
- 236,935