Not to be confused with 2C-B (sometimes also referred to as "bromomescaline")
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
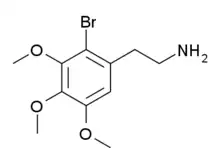
| Formula | C11H16BrNO3 |
| Molar mass | 290.157 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
2-Bromomescaline (2-Br-M) is a derivative of the phenethylamine hallucinogen mescaline which has an unusual 2-bromo substitution.[1][2] It is an agonist for serotonin receptors, with a binding affinity of 215 nM at 5-HT1A, 513 nM at 5-HT2A and 379 nM at 5-HT2C, so while it is around ten times more tightly binding than mescaline at 5-HT1A and 5-HT2A receptors, it is over twenty times more potent at 5-HT2C.[3]
See also
References
- ↑ Shulgin A, Manning T, Daley PF (2011). The Shulgin Index. Volume 1. Psychedelic Phenethylamines and Related Compounds. Transform Press. p. 280–281, 355. ISBN 978-0-9630096-3-0.
- ↑ Pecherer B, Sunbury RC, Brossi A (June 1972). "A novel synthesis of aromatic methoxy and methylenedioxy substituted 2,3,4,5-tetrahydro-1H3-benzazepines". Journal of Heterocyclic Chemistry. 9 (3): 609–616. doi:10.1002/jhet.5570090322.
- ↑ Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine Von der Struktur zur Funktion. Nachtschatten Verlag AG. pp. 908–910. ISBN 978-3-03788-700-4.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.