 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.153 |
| Chemical and physical data | |
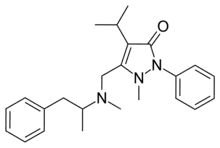
| Formula | C24H31N3O |
| Molar mass | 377.532 g·mol−1 |
| 3D model (JSmol) | |
| |
Famprofazone (Gewodin, Gewolen) is a nonsteroidal anti-inflammatory agent (NSAID) of the pyrazolone series which is available over-the-counter in some countries such as Taiwan.[1][2][3] It has analgesic, anti-inflammatory, and antipyretic effects.[1][2] Famprofazone has been known to produce methamphetamine as an active metabolite, with 15–20% of an oral dose being converted to it.[4][5] As a result, famprofazone has occasionally been implicated in causing positives on drug tests for amphetamines.[3]
See also
References
- 1 2 Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. p. 1932. ISBN 3-88763-075-0.
- 1 2 Hall JA, Morton I (1999). Concise dictionary of pharmacological agents: properties and synonyms. Kluwer Academic. p. 342. ISBN 0-7514-0499-3.
- 1 2 Chan KH, Hsu MC, Tseng CY, Chu WL (2010). "Famprofazone use can be misinterpreted as methamphetamine abuse". Journal of Analytical Toxicology. 34 (6): 347–353. doi:10.1093/jat/34.6.347. PMID 20663288.
- ↑ Oh ES, Hong SK, Kang GI (March 1992). "Plasma and urinary concentrations of methamphetamine after oral administration of famprofazone to man". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 22 (3): 377–384. doi:10.3109/00498259209046649. PMID 1496827.
- ↑ Shin HS, Park BB, Choi SN, Oh JJ, Hong CP, Ryu H (1998). "Identification of new urinary metabolites of famprofazone in humans". Journal of Analytical Toxicology. 22 (1): 55–60. doi:10.1093/jat/22.1.55. PMID 9491970.
| pyrazolones / pyrazolidines | |
|---|---|
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams |
|
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
Key: underline indicates initially developed first-in-class compound of specific group; #WHO-Essential Medicines; †withdrawn drugs; ‡veterinary use. | |
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| CARTooltip Constitutive androstane receptor |
|
|---|---|
| PXRTooltip Pregnane X receptor |
|
| |
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.