 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
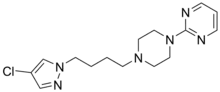
| Formula | C15H21ClN6 |
| Molar mass | 320.83 g·mol−1 |
| 3D model (JSmol) | |
| |
Lesopitron (E-4424) is a selective full agonist of the 5-HT1A receptor which is structurally related to the azapirones.[1] In 2001 it was under development by Esteve as an anxiolytic for the treatment of generalized anxiety disorder (GAD).[2][3] It made it to phase II clinical trials but was apparently discontinued as no new information on lesopitron has surfaced since.[2][3]
See also
References
- ↑ Haj-Dahmane S, Jolas T, Laporte AM, et al. (April 1994). "Interactions of lesopitron (E-4424) with central 5-HT1A receptors: in vitro and in vivo studies in the rat". European Journal of Pharmacology. 255 (1–3): 185–96. doi:10.1016/0014-2999(94)90097-3. PMID 8026543.
- 1 2 Micheli F (February 2001). "Lesopitron (Esteve)". IDrugs: The Investigational Drugs Journal. 4 (2): 218–24. PMID 16032484.
- 1 2 Fresquet A, Sust M, Lloret A, et al. (February 2000). "Efficacy and safety of lesopitron in outpatients with generalized anxiety disorder". The Annals of Pharmacotherapy. 34 (2): 147–53. doi:10.1345/aph.19041. PMID 10676820.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.