Of course there are. That's why we solve the time-independent version of the Schrödinger equation to begin with: because given any eigenfunction $\psi_0$ of the hamiltonian with eigenvalue $E$, the phase-evolved combination
$$
\psi(t) = e^{-iEt/\hbar}\psi_0
$$
is a solution of the time-dependent Schrödinger equation, and, moreover, any linear combination of such solutions is still a solution.
There is, of course, an understandable prejudice against taking a stationary state as an initial condition for the TDSE (but it is just a human prejudice with no real meat to back it up). If that really bothers you, then you can just take a nontrivial linear combination, like, say,
$$
\psi = \frac{\psi_{100}+\psi_{210}}{\sqrt{2}},
$$
and it will then show oscillations in both the position-space and momentum-space probability distributions. To borrow from my answer to Is there oscillating charge in a hydrogen atom?, the explicit wavefunction is given by
$$
\psi(\mathbf r,t)
= \frac{\psi_{100}(\mathbf r,t) + \psi_{210}(\mathbf r,t)}{\sqrt{2}}
=
\frac{1}{\sqrt{2\pi a_0^3}}
e^{-iE_{100}t/\hbar}
\left(
e^{-r/a_0}
+
e^{-i\omega t}
\frac{z}{a_0}
\frac{
e^{-r/2a_0}
}{
4\sqrt{2}
}
\right)
,
$$
and this goes directly into the oscillating density:
$$
|\psi(\mathbf r,t)|^2
=
\frac{1}{2\pi a_0^3}
\left[
e^{-2r/a_0}
+
\frac{z^2}{a_0^2}
\frac{
e^{-r/a_0}
}{
32
}
+
z
\cos(\omega t)
\,
\frac{e^{-3r/2a_0}}{2\sqrt{2}a_0}
\right]
.
$$
Taking a slice through the $x,z$ plane, this density looks as follows:
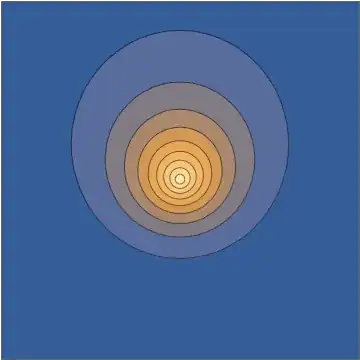
This combination gives you an explicit analytical solution of the time-dependent Schrödinger equation. Now, again, it is understandable to dismiss this as somehow "not being a real wavepacket", partly because from some perspectives it might feel "too easy", but all of those are human prejudices with very little support on well-defined and truly meaningful mathematical criteria on the initial conditions or the corresponding solution. This is an honest-to-goodness electronic wavepacket moving under the influence of a point-charge nucleus.
