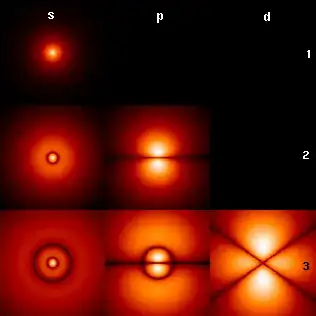
Probability densities are illustrated in text books and on Wikipedia as static pictures. Is the probability density of an electron within an isolated Hydrogen atom static or does it oscillate in some way?
Asked
Active
Viewed 374 times
1 Answers
1
In this link, one can see how the plots you show are derived from the solution of the Schrodinger equation for the hydrogen atom. There are checkable options.
There is no time dependence in the wavefunctions, or the corresponding probability distributions for the given eigenvalues (seen by checking next to the solutions).
Emilio Pisanti has given here the case of the time dependent hydrogen atom solutions:
and it will then show oscillations in both the position-space and momentum-space probability distributions.
anna v
- 236,935