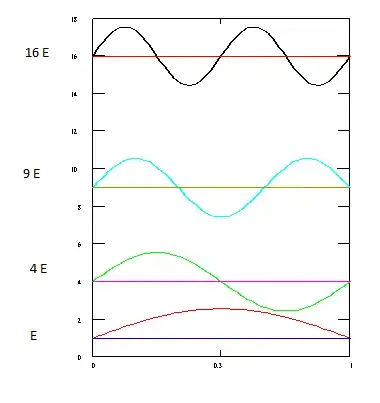
Here is a picture of the energy states of infinite potential well.
We can see That the first level have a half wavelength which fittes with a full wave of the second level. 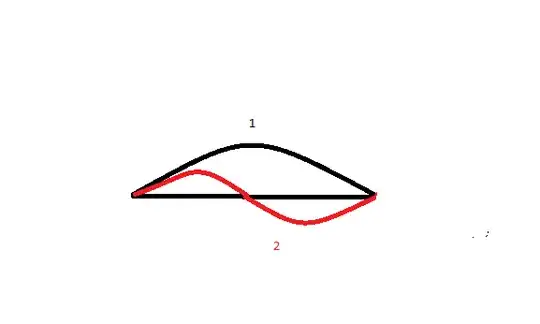
$$\frac{ \lambda _{1} }{2} = \lambda _{2}$$
$$ \Rightarrow \lambda _{1} =2 \lambda _{2}$$
$$ \Rightarrow \frac{1}{E_{1} } = \frac{2}{E_{2} } $$
$$\Rightarrow E_{2} = 2E_{1} $$
We have used the formula:$$E=h \nu $$
$$\Rightarrow E= \frac{hc}{ \lambda } $$
We started with our conception of classical waves, we used Planck's relation $E=h \nu $ and we have concluded $E_{2} = 2E_{1} $. Which we used to know wrong.
We know that $$E_{n}= n^{2} E_{1}$$
Now we start from here:
$$E_{2}= 4 E_{1}$$
$$ \Rightarrow \frac{1}{ E_{1} } = \frac{4}{ E_{2} } $$
$$ \Rightarrow \lambda _{1} = 4 \lambda _{2} $$
$$\Rightarrow \frac{ \lambda _{1} }{2} =2 \lambda_{2} $$
We can represent the last line with a picture
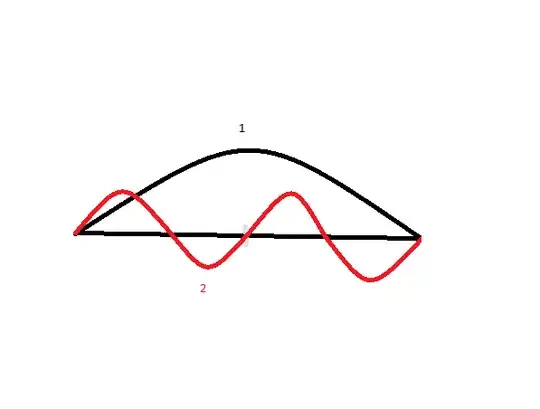
Here two waves fittes in a half wavelength of the first level.
Now my question is why our concept of classical waves doesn't reconcile with the energy level wave functions? Is there any other difference between classical and quantum wave? What's wrong in my analysis?