 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Phenylethan-1-amine | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.588 |
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H11N | |
| Molar mass | 121.183 g·mol−1 |
| Density | 0.94 g/mL |
| Melting point | -65 C |
| Boiling point | 187 °C (369 °F; 460 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Corrosive |
| Related compounds | |
Related stereoisomers |
(R)-(+)- (CAS [3886-69-9]) (S)-(–)- (CAS [2627-86-3]) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
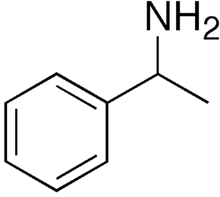
1-Phenylethylamine is the organic compound with the formula C6H5CH(NH2)CH3. This primary amine is a colorless liquid is often used in chiral resolutions. Like benzylamine, it is relatively basic and forms stable ammonium salts and imines.
Preparation and optical resolution
1-Phenylethylamine may be prepared by the reductive amination of acetophenone:[1]
- C6H5C(O)CH3 + NH3 + H2 → C6H5CH(NH2)CH3 + H2O
The Leuckart reaction, using ammonium formate, is another method for this transformation.[2]
L-malic acid is used to resolve 1-Phenylethylamine, a versatile resolving agent in its own right. The dextrorotatory enantiomer crystallizes with the malate, leaving the levorotatory form in solution.[3]
See also
References
- ↑ John C. Robinson, Jr. and H. R. Snyder (1943). "α-Phenylethylamine". Organic Syntheses. 23: 68. doi:10.15227/orgsyn.023.0068.
- ↑ Mann, F. G.; Saunders, B. C. (1960). Practical Organic Chemistry, 4th Ed. London: Longman. pp. 223–224. ISBN 9780582444072.
- ↑ A. W. Ingersoll (1937). "d- and l-α-Phenylethylamine". Organic Syntheses. 17: 80. doi:10.15227/orgsyn.017.0080.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.