The radial Schrodinger equation for the hydrogen atom is given by: $$\frac{1}{R}\frac{d}{dr}\left[r^2 \frac{dR}{dr}\right]+\frac{2m_e}{\hbar^2}\left(Er^2+ke^2r\right)=l(l+1).$$ Following the Bohr model we substitute the energy $E$ and radius $a_0$ given by $$E = -\frac{(ke^2)^2 m_e}{2\hbar^2 n^2},$$ $$a_0 = \frac{\hbar^2}{k e^2 m_e}.$$ We also change variable $r \rightarrow a_0\ r$ to give the following dimensionless radial equation: $$r^2 R''+2rR'-\frac{r^2R}{n^2}+2rR-l(l+1)=0.$$ The standard hydrogen groundstate is $n=1$ and $l=0$.
Instead we try $n=1/2$ and $l=0$.
WolframAlpha gives a solution of the form: $$R(r)=e^{-2r}\ U(\frac{1}{2},2,4r),$$ where $U(a,b,x)$ is the confluent hypergeometric function of the second kind.
The wavefunction $R(r)$ is badly behaved at $r=0$ with the real part diverging and a discontinuity in the imaginary part.
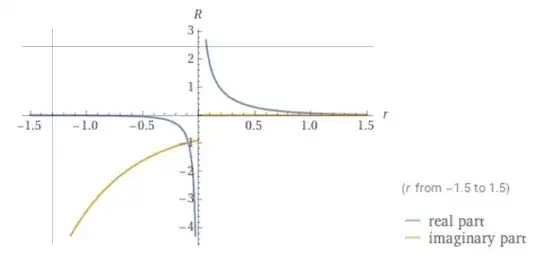
But in order to calculate the radial probability density which is actually observable we just need a function of the form:
$$p(r) = 4 \pi r^2 |e^{-2r}\ U(\frac{1}{2},2,4r)|^2.$$
This seems well-behaved at $r=0$ and $r\rightarrow\infty$.
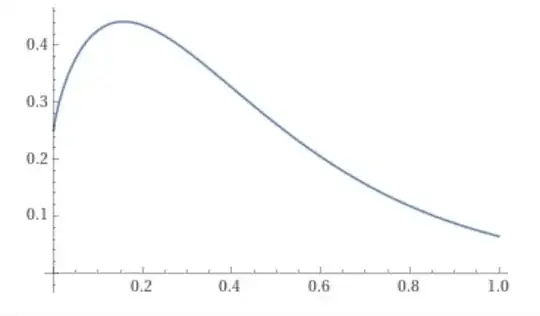
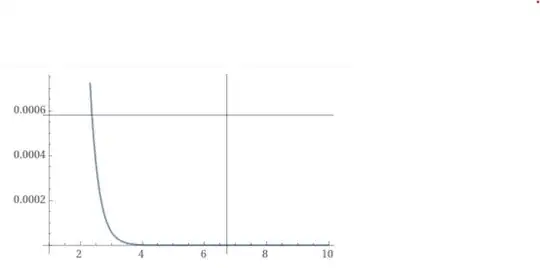 WolframAlpha says:
$$\lim_{r\rightarrow 0^+}4\pi r^2|e^{-2r}\ U(\frac{1}{2},2,4r)|^2=\frac{1}{4}.$$
Therefore is $n=1/2$, $l=0$ a valid hydrogen wavefunction?
WolframAlpha says:
$$\lim_{r\rightarrow 0^+}4\pi r^2|e^{-2r}\ U(\frac{1}{2},2,4r)|^2=\frac{1}{4}.$$
Therefore is $n=1/2$, $l=0$ a valid hydrogen wavefunction?