In case of rusting of iron the chemical reaction is not fast enough. The oxygen used is not molecular oxygen from the atmosphere but it is the oxygen from water molecule. The reaction is not rapid and appreciable amount of heat is also not produced. Also we do not associate any ignition temperature with this reaction. So can we say rusting of iron is not a combustion reaction?
3 Answers
Rusting of iron is an oxidation reaction, but not combustion. Although the reaction's equation looks the same as the equations for combustion (e.g., of hydrogen and oxygen mixture, $2\text{H}_2+\text{O}_2\to2\text{H}_2\text{O}$), the reaction mechanism is different: combustion reactions are chain reactions, where intermediate reaction products (e.g., various radicals of hydrogen, oxygen and water molecules in the example of $2\text{H}_2+\text{O}_2\to2\text{H}_2\text{O}$) serve to trigger more and more reactions, resulting in positive feedback / avalanche process, which is the reason for the rapidity of combustion and explosions in comparison to iron oxidation, which can be essentially treated as encounter between two molecules.
The figure for a chain reaction below comes from here
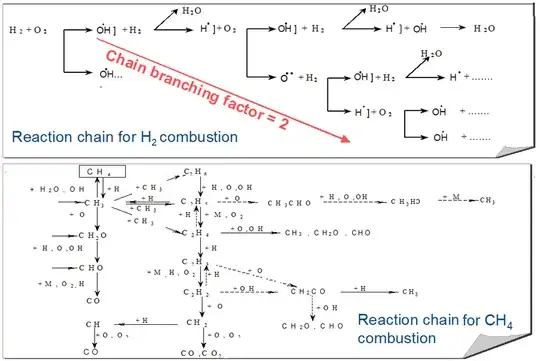
References:
- Introduction to Physics and Chemistry of Combustion: Explosion, Flame, Detonation by Michael Liberman.
Rusting of iron does not fulfill the definition of combustion, and is not combustion, just exothermic oxidation. For instance, combustion reactions are characterized by high temperature, usually several hundred to several thousands degrees Celsius, whereas rusting of iron usually does not result in any noticeable change from room temperature. Even in the near optimal case, like iron-based heat packs that generate heat from powdered iron reacting with air, the reaction temperature does not even reach 100 degrees Celsius. I guess you could still call rusting a combustion reaction, you do you. However, calling it combustion does not make it a combustion reaction.
- 11
We can call rusting of iron a combustion reaction. Water is needed in the reaction to carry the ionic current in the short circuited electrochemical cell reaction.