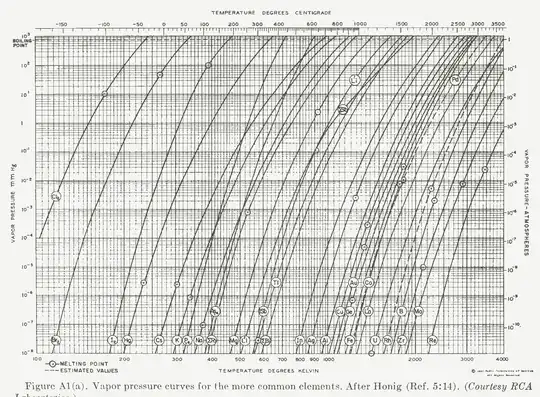
When we look at the phase diagram of a material, at low enough temperature and pressure, it tends to sublimate:
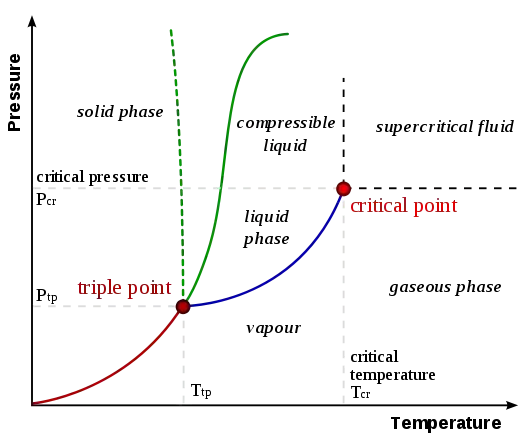 So can it be true that no matter how strong a solid is, if at a low enough temperature, I maintain pressure below a certain level, it is bound to sublimate.
So can it be true that no matter how strong a solid is, if at a low enough temperature, I maintain pressure below a certain level, it is bound to sublimate.
But is this true for all solids, no matter how strong the bonding is and no matter how much energy has been lowered by formation of solid?? on Wikipedia page, even common metals seem to sublimate (https://en.wikipedia.org/wiki/Materials_for_use_in_vacuum)
Suppose I consider a simple chain with nearest neighbour hopping, the electrons occupy the valence band with energy as $-2t_0(1-\cos(ka))$ where $t_0$ is nearest neighbour hopping, this total energy is negative hence shouldn't the solid be always energetically favourable than separate atoms roaming in space (if phonons are not energetic enough to break the chains)??
I am thinking taking into account the thermodynamics of photons and phonons is also important but I am not able to think of their role in this