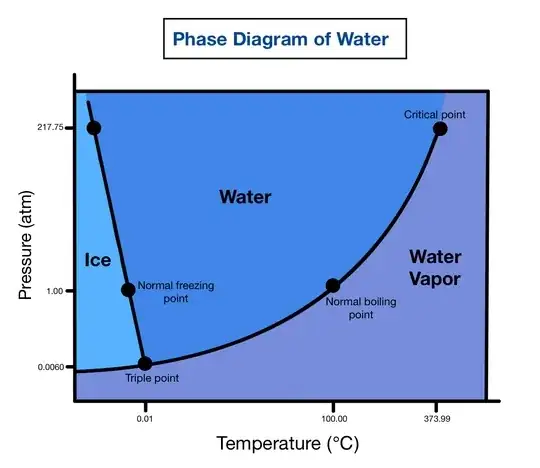
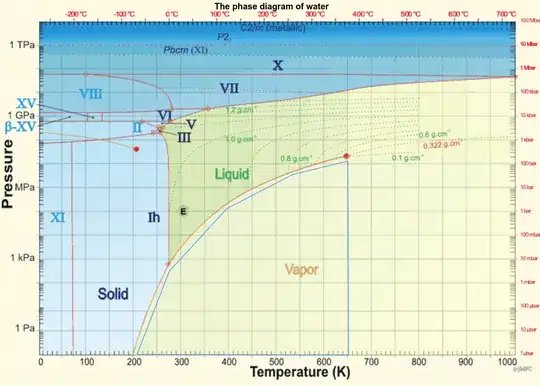
Naively, when the water cools down to low temperature, the water goes to the ice solid phase. (Like below 0 celsius at 1 ATM pressure.)
Can water remain in the gas form at zero Kelvin (0K) temperature, when the pressure is low enough?
Namely,
- Can the water vapor (gas) phase persist at a zero Kelvin (0K) temperature, when the pressure is low enough?
According to the first phase diagram in Celsius, it cannot tell.
According to the second phase diagram in Kelvin to near 0K, it seems the answer is no.
- What is the nature of the quantum phase transition at zero Kelvin (0K) between the water solid (ice) and the water gas phase (vapor)? (the first or second order or continuous higher phase transitions, Ginzburg-Landau potential, etc.)