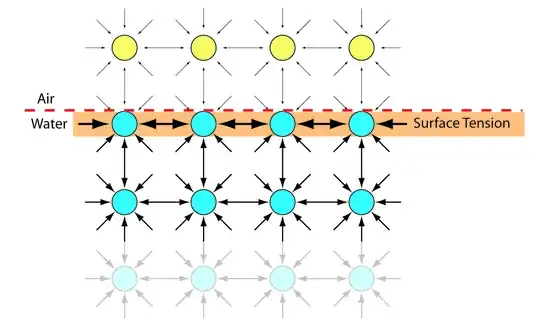
I understand that surface tension arises at the liquid-vapour interface due to the asymmetric nature of long-range attractive forces and the short-range repulsive forces acting on the interface where there exists a gradient of density as we go from the liquid phase to the vapour. As explained by Michael Berry[1], the short-range repulsive interaction, which is proportional to the density, decreases faster compared to the long-range attractive force, when going from the bulk to the vapour.
But I don't understand how the surface tension arises in the case of a solid-liquid interface. Solid particles are tightly bound to each other and I don't think that there exists a smooth density gradient at the solid-liquid interface. So, how does a surface tension force which is parallel to the interface arise in this case?
Same question for the solid-vapour interface. I'm not even sure if there can exist a surface tension force parallel to the solid-vapour interface; apparently the 'surface tension' force is just a reaction force from the surface molecules as a response to deformation.[4]
I would prefer an explanation in terms of molecular forces rather than in terms of thermodynamic arguments. Thermodynamic arguments can prove that if the system adopts some configuration, then it is stable there. But this does not shed any light on the actual mechanism of how the system adopts the configuration, in mechanical terms of forces.
I've read the following references but have failed to understand the solid-liquid and solid-vapour cases.
- Berry, Michael V. ‘The Molecular Mechanism of Surface Tension’. Physics Education, vol. 6, Mar. 1971, pp. 79–84. NASA ADS, doi: 10.1088/0031-9120/6/2/001. PDF (available from author's website): https://michaelberryphysics.files.wordpress.com/2013/07/berry018.pdf.
- Why is surface tension parallel to the interface? https://physics.stackexchange.com/q/150853.
- Marchand, Antonin, et al. ‘Why Is Surface Tension a Force Parallel to the Interface?’. American Journal of Physics, vol. 79, no. 10, Sept. 2011, pp. 999–1008. aapt.scitation.org (Atypon), doi: 10.1119/1.3619866. arXiv: https://arxiv.org/abs/1211.3854.
- Makkonen, Lasse ‘Misinterpretation of the Shuttleworth equation’ Scripta Materialia, vol. 66, no. 9, 2012, pp. 627-629, doi: 10.1016/j.scriptamat.2012.01.055. PDF: https://cris.vtt.fi/en/publications/misinterpretation-of-the-shuttleworth-equation.
Edit:
My understanding of the solid-liquid surface tension is as follows. Please correct me if it is incorrect.
Liquid particles near the solid surface are attracted towards the solid surface and thus there is a slight increase in liquid density near the interface. This increased density causes increased pressure and this pressure gives rise to a tangential force in the liquid at the solid-liquid interface. But this force is not equal to the solid-liquid surface tension. This is what is written in the third reference. I don't understand why this is the case.
Second edit:
A new pre-print claims, if I understand correctly, that the downward acting force in Young's diagram, opposite to $\gamma_\text{lv}\sin\theta$, is due to vaccum polarization. $\gamma_\text{lv}$ is the liquid-vapor surface tension, and $\theta$ is the contact angle. References:
- Rouslan Krechetnikov and Andrei Zelnikov ‘Vacuum polarization at the tip of a water drop’. arXiv: https://arxiv.org/abs/2408.05833v1.
- Rouslan Krechetnikov and Andrei Zelnikov ‘Nonretarded limit of the Lifshitz theory for a wedge’ Physical Review A, vol. 105, issue 2, Feb 2022, American Physical Society. doi: 10.1103/PhysRevA.105.022802. arXiv: https://arxiv.org/abs/2103.16715.
- Rouslan Krechetnikov and Andrei Zelnikov ‘Scarf for Lifshitz’ European Physical Journal Plus, vol. 136, issue 7, July 2021. Springer Science and Business Media LLC. doi: 10.1140/epjp/s13360-021-01714-3. arxiv: https://arxiv.org/abs/2103.16713.