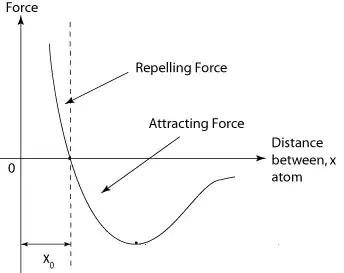
The classic picture for intermolecular forces looks something like this:
https://en.wikipedia.org/wiki/Lennard-Jones_potential#/media/File:12-6-Lennard-Jones-Potential.svg
Unless two molecules are very close to each other, the forces between them are generally attractive(In the photo above, for distances greater than $r_{m}$). This is due primarily to the Van der Waals force.
Molecules well inside the liquid, on average experience the Van der Waals attraction from all sides. Hence the net such force on such molecules is zero. But for molecules near the surface, there is an attraction only towards the bulk of the liquid. Hence, the liquid attempts to minimize it's surface area, and this is termed as surface tension.
Edit: When you talk about 'pressure', it is when the short range repulsion forces come into play when two molecules are very close to each other. For example, when a liquid molecule comes very close to molecules of the container, it rebounds. The same thing is happening for two molecules coming very close to each other either near the surface or within the bulk of the liquid. But the bulk attractive Van Der Waals forces have a more dominating effect for molecules near the surface, when compared with the repulsive 'bounces' with molecules near the surface.