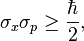To summarize, assume hypothetically we managed to find a way in the future where we can have a look at an electron without disturbing it by measurement or causing its wave function to collapse, would the uncertainty principle still hold in such a case?? Why/Why not?
To start with any measurement when looked at the quantum mechanical level involves interactions, i.e. at least a photon exchange with the electromagnetic field of the particle under observation. So the thought experiment per se is not valid.
There does exist the possibility of measuring the electron's position and momentum in a non destructive way with soft photons interacting without destroying much the original momentum and position in space, within measurement errors.
And we come to the difference between measurement errors and the Heisenberg uncertainty principle. The error in measuring the momentum of the electron, in this bubble chamber photo of an electron positron pair

comes from the measurement error of the curvature in the magnetic field and the addition in quadrature of the magnetic field error . The more points fitted to the curve in the chisquare fit the smaller the curvature error. It is the measurement on one electron. The x, y,z position of the start of the electron also has corresponding measurement errors .
The Heisenberg Uncertainty Principle for this measurement tells us the following:
Now that you know the momentum p_x, you cannot know the x better than
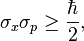
In the photo above, the HUP is fulfilled because our measurements in space cannot be better than microns and on momenta than fractions of an MeV . h_bar is a very small number and for classical sizes can be considered zero.
In this link some numbers are given on how the HUP once the momentum is known constrains the range of location in space for particles in a beam, where the numbers are small enough that the size of h_bar becomes important.
One has to keep in mind that in contrast to the nice classical curve in the photo above , the trajectory of the electron is not described by a single function. What can be described as a single function is the probability of finding an electron in a specific momentum and position, which is given by the wave function, a solution of the quantum mechanical equations.
The HUP reflects this uncertainty due to the probabilistic nature of quantum mechanics. The restrictions it imposes are useful for estimating behaviors and lifetimes etc without solving explicitly a potential problem equation and applying boundary conditions.
The HUP is the manifestation of the commutator relations which are fundamental in the structure of quantum mechanics as a theory. The uncertainty comes because the momentum and position operators do not commute. This means that the two operators cannot both display eigenvalues, i.e. accurate predictions, at the same time. This last is true for all observables from pairs of non commuting operators.