Summary: The hydrogen atom has a single electron distribution, regardless of which coordinate system is used to solve the Schrödinger equation. Note that the author is plotting the wavefunction after conversion to Cartesian system. A wave function expressed in any coordinate system should yield the same probability density for finding the electron around the nucleus. Why, then, would one accept different probability densities for orbitals obtained in different coordinate systems?
This question is related to a recent open access article (Orbitals in General Chemistry: Mathematical Realities). The authors show that the common atomic orbital shapes taught in textbooks, like a sphere for s, dumb bell shape for p orbitals, and various clover shape for the d orbitals result from solving the Schrödinger Equation in spherical coordinates.
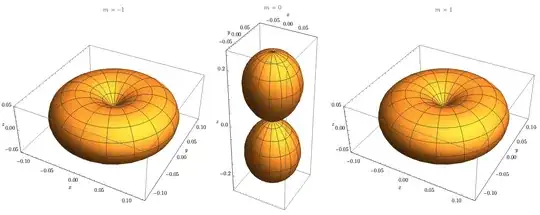
Lamoureux and Ogilvie then go on to discuss that exactly the same hydrogen-atom time independent Schrödinger equation can be solved in four alternative separations in paraboloidal, prolate spheroidal (ellipsoidal), and spheroconical coordinates—each yielding its own complete set of closed-form amplitude functions (“orbitals”) with distinct quantum-number labels.
Despite their very different shapes and quantum-number schemes (I show two examples below), all of these functions should describe identical physical states, demonstrating that orbitals are really just coordinate-dependent basis functions rather than uniquely real physical entities. This multiplicity calls into question the physical meaning the scientists ascribe to any particular orbital representation.
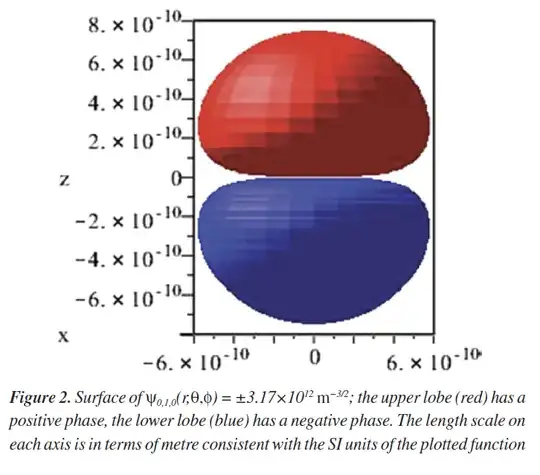
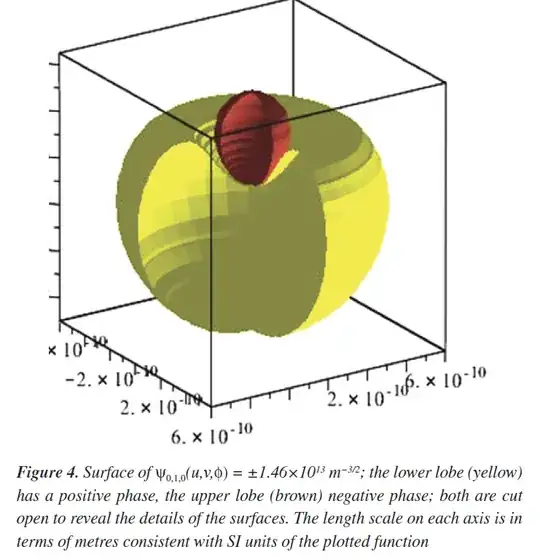
Question: Now nature does not change the reality based on the human's invention of coordinate system and hydrogen atom is unaware of the choice coordinate system. If these various coordinate‐system “orbitals” are just different basis functions for the same physical eigenstates, how do we reconcile their radically different individual shapes with the fact that the underlying probability density of the electron in the H-atom $|\Psi|^2$ must be the same? In particular, how would we demonstrate (analytically or numerically) that $|\Psi_{0,1,0}|^2$ computed in any of these coordinate systems yields the identical 3D electron‐density distribution? The figure will show the different shape in different coordinate systems.
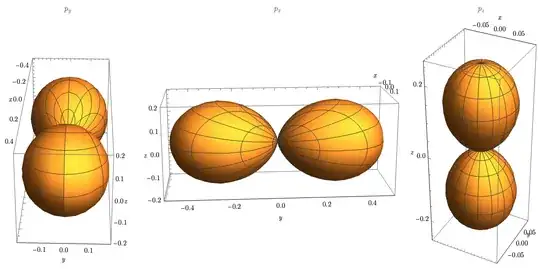
For example, most readers can recall the p-orbital shape from spherical coordinates.
however, the orbital from paraboloidal coordinate system, we get this shape from the same paper linked above.