Consider transition of an electron from higher orbit to some lower orbit. For simplicity just assume electron in an hydrogen atom. Through transition, it emits radiation which is equal to the energy difference between the orbits. The frequency of the radiation is just the energy over Planck's constant. Now when we do find this frequency, how do we know that this was the actual frequency of the radiation that was released by the electron and observed in the detector. Because for a detector, the electron is never stationary. In simple terms, doppler effect should come into picture and the electron when it makes the transition should be considered in motion. Even if the transition was instantaneous, there should be some delay or kink in the radiation because of the doppler shift. In this way the frequency of the emitted radiation should be different than the frequency that was detected. Please enlighten me about this, because I am guessing that if that it is a genuine question, it might arrive from yet another consequence of the uncertainty principle.
2 Answers
If I have understood you correctly you're thinking that because the electron is orbiting the nucleus it will be alternately heading towards and away from the detector as it orbits, and this should cause a doppler shift of the light - if this isn't what you meant ignore this answer!
Anyhow, the answer is that the electron is not orbiting the nucleus like a planet orbits a star. You have probably been taught the Bohr model of the atom, and the Bohr model does indeed describe the atom this way. However the Bohr model is completely wrong in this respect. Electrons in atoms are not little points whizzing around the atom - they are more like fuzzy clouds spread out over the atom and they do not move. The $1s$ electron is a cloud shaped like the $1s$ orbital, and the $2p$ electron is a cloud shaped like the $2p$ orbital. So the $2p ⟶1s$ transition is actually just the electron "cloud" changing shape.
The result is that there is no Doppler shift associated with the motion of the electron. We do see broadening of the emission due to other factors, but not due to any motion of the electron.
- 367,598
Indeed, this is how laser cooling/Doppler cooling works - with a correction that the momentum is transferred not specifically to electron, but to the atom as a whole (this is easily forgotten in discussion of electron transitions, which are usually analyzed in the center-of-mass reference frame, although good QM texts usually spell this step.)
Thus, an atom moving towards a laser absorbs higher frequency, but then re-emits the absorbed light (on average) at the frequency corresponding to the peak in its spectral line, thus losing some momentum. Likewise an atom moving away from the laser absorbs lower frequency and has its (negative) momentum increased.
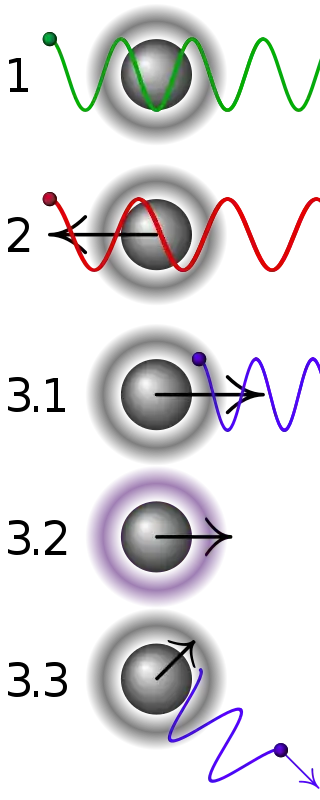
Simplified principle of Doppler laser cooling:
1 A stationary atom sees the laser neither red- nor blue-shifted and does not absorb the photon.
2 An atom moving away from the laser sees it red-shifted and does not absorb the photon.
3.1 An atom moving towards the laser sees it blue-shifted and absorbs the photon, slowing the atom.
3.2 The photon excites the atom, moving an electron to a higher quantum state.
3.3 The atom re-emits a photon but in a random direction. The atom momentum vectors would add to the original if they were in the same direction but they are not so the atom has lost energy and, therefore, cooled.
- 68,984