This is a very interesting question and since I have thought before about it, I would like to share my answer.
As far as I am concerned, there has been no empirical evidence of coexistence between a fully ionized plasma and a neutral gas as separated phases in contact such as ice and water at $0^{\circ}$C. As @Gotaquestion correctly pointed out, the transition between gaseous and plasma state is continuous and gradual.
However, the heat capacity at constant volume, $C_{V}$, and also the heat capacity at constant pressure, $C_{p}$, exhibit peak values in some temperature intervals where atom/molecule ionization due to energy exchange gets more probable. In the figure below, extracted from an old paper form Phys. Fluids by Drellishak et al, $C_{V}(T)$ and $C_{p}(T)$ curves were calculated using thermodynamics principles and the partition function of diatomic nitrogen and diatomic oxygen.
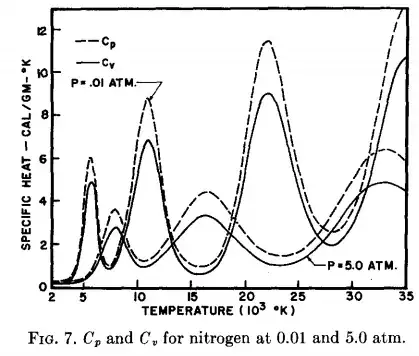
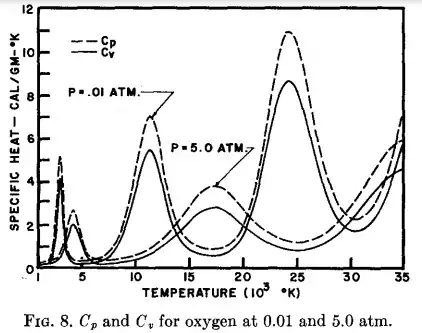
In these figures, the peaks show the effect of very strong increase in the specific heats mainly due to energy loss by electron ionization. After each peak, the curve decreases to a minimum which is always higher than the previous. This happens because after a peak occurs, the corresponding ionized electrons are introduced in the system, giving their own contribution to the specific heat by increasing the degrees of freedom of the system.
Note that as the peaks get narrower they should resemble more and more a second order phase transition. Second order phase transitions are characterized by continuous $G(T, P), S(T,P), V(T,P)$, but discontinuous constant pressure heat capacity $C_{p}(T,P)$ (a good reference may be find here).
Finally, I must point some caveats in this answer. Here I considered what is called "classic plasma", which uses classical statistical mechanics in the treatment of Debye-Hückel.
I am not a specialist in quantum mechanical plasmas, but maybe in such systems other kind of phase transition may occur.