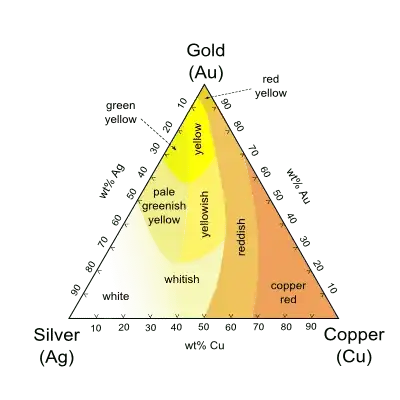
There are various issues at play here to discuss.
To begin with the simple examples, copper, silver, and gold are all fcc metals with a nearly perfect spherical Fermi surface for the electrons. This results in two things: the electronic (and hence optical) properties are isotropic, and it is reasonable to consider the conduction electrons as a nearly perfect electron gas.
Considering the interactions of an electron gas with light, the key parameter is the plasma frequency, the natural harmonic frequency that the electron gas can slosh around. To a first approximation this is given by:
$$\omega_{p}^{2} = {4 \pi n e^{2} \over m}$$
with $n$ the number density of free electrons and $m$ the effective mass of the electron in the band structure of interest.
One can now consider the electron gas as a Lorentz oscillator - for incident light below the plasma frequency the electrons readily respond to the time varying field and the metal readily reflects there. Above the plasma frequency the electrons can no longer respond and light penetrates the metal and reflectivity is reduced there. This fairly simple theory explains the response of copper, silver, and gold. For copper and gold the plasma frequency is in the visible range, and the reflectivity in the blue and green is reduced so they look reddish. If you could see into the near UV you could see the silver reflectivity drop there.
Some comments and answers bring up osmium, which is a good example of where the simple plasma frequency model runs into trouble for many different reasons. The first reason is straightforward - osmium has an hcp crystal structure so we expect different electrical and optical behavior in different directions (within the basal plane, and perpendicular to the basal plane). As one example of a non-isotropic hcp electronic structure I'll point to my answer to this question for the hcp beryllium electronic structure. There the 'cigar' structure is the Fermi surface for holes and the 'coronet' is the Fermi surface for electrons - yes, the conducting charge in the basal plane is different from the conducting charge perpendicular to the basal plane, as confirmed by Hall measurements in the different crystal directions.
Now, what is happening with osmium? First, like beryllium, it turns out that there is both electron and hole contributions to the conductivity, each having separate Fermi surface. And, since osmium has way more electrons, the electronic structure is way more complicated. Fortunately, the Fermi surface has been measured (Kamm and Anderson, Phys Rev B 2(8) 2944 (1970)). Figure 7 from the paper shows the electron Fermi surfaces:
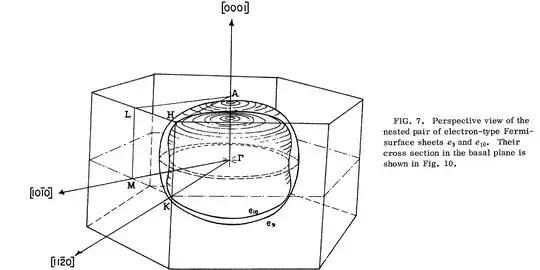
Yes, two surfaces, neither terribly spherical, nested one inside the other.
For completeness sake, Figure 12 is the hole Fermi surface:
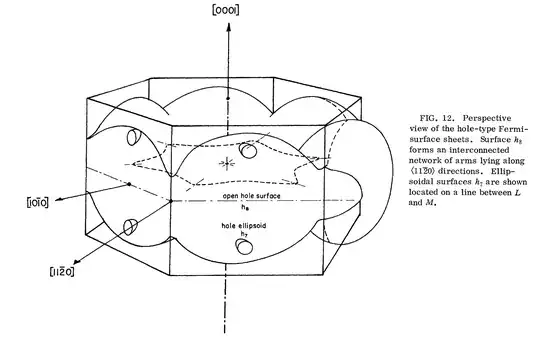
But, why is osmium "blue". The answer lies in those nested electron Fermi surfaces. This structure allow interband transitions from one Fermi surface to the other, and it turns out that these occur primarily below the blue, leaving a strong impression of blue for the color. More on the optical response can be found in Nemoshkalenko et al., Sov. Phys. JETP 63(1) 115 (1986).
So, yes, osmium does not behave like copper, silver, or gold. The differences highlight the need to often consider details of the crystal structure and the resulting electronic structure, Fermi surfaces, and their impact on optical properties.