This is certainly an interesting question, but it may be based on a false premise.
Despite the claim by Wikipedia and many other online sources, it does not appear to be true that ambient-pressure steaming of food is a consistently faster cooking method than boiling it.
Cooking times seem more limited by thermal conductivity within the food item than the difference in surface heat transfer between boiling and steaming.
If we only count the time after the food item is placed in the boiling water or steam, then according to the (admittedly sparse) research literature, steaming is usually slower than boiling.
If, however, we include the time taken to heat the water to boiling (as noted in @Solomon-Slow's comment), there is no question that steaming food is usually faster overall. It takes much less time to boil the smaller amount of water needed to make sufficient steam than to boil the large amount of water needed to cover food items. For example, it might only take 4 minutes to boil the 500 mL of water needed to steam a pot of potatoes, but almost 20 minutes to boil the 4 L of water needed to cover the potatoes. Similarly, boiling broccoli might take 3 minutes compared to 4 minutes for steaming, but this ignores the 4 minutes needed to heat the water for boiling which makes a total of 7 minutes for boiling from a cold start.
For more discussion of the relevant physics, see my question and answer: "Why is steaming food not faster than boiling it?".
Optimal cooking times for boiling versus steam from the research literature
Given that cooking times are important for food safety, I was surprised how hard it was to find cooking times in the research literature for boiling vs steaming . Below are cooking times (in minutes) that I found. The shorter of the steaming and boiling times are in boldface; differences $\leq 10$% are considered a tie.
$$
\begin{align}
&Vegetable& &\quad Boiling &&Steam &&Source \\
&\mathrm{Asparagus}& &\quad\mathbf{ 2.5}&& 6 &&\mathrm{DanowskaOziewicz2020}\\
&\mathrm{Beans\ (green)}& &\quad\mathbf{15 }&& 17 &&\mathrm{Warthesen1984} \\
&\mathrm{Broccoli}& &\quad\mathbf{ 3 }&& 4 &&\mathrm{Bongoni2014} \\
&& &\quad\mathbf{ 5 }&& 10 &&\mathrm{Lee2018} \\
&& &\quad\mathbf{ 7 }&&\mathbf{ 7}&&\mathrm{Rennie2010} \\
&& &\quad\mathbf{ 7.5}&&\mathbf{ 8}&&\mathrm{DanowskaOziewicz2020}\\
&& &\quad 8 &&\mathbf{ 7}&&\mathrm{Warthesen1984} \\
&& &\quad\mathbf{ 8 }&& 13 &&\mathrm{Pellegrini2010} \\
&& &\quad\mathbf{10 }&&\mathbf{10}&&\mathrm{TorresdeCastro2020} \\
&\mathrm{Blue\;Crab}& &\quad\mathbf{ 5 }&& 7 &&\mathrm{Hazard2010} \\
&\mathrm{Brussels\ sprouts}& &\quad\mathbf{10 }&& 17 &&\mathrm{Pellegrini2010} \\
&\mathrm{Cabbage}& \mathrm{shredded}&\quad\mathbf{ 5 }&& 10 &&\mathrm{Rennie2010} \\
& &\mathrm{wedge}&\quad\mathbf{10 }&&\mathbf{10}&&\mathrm{Warthesen1984} \\
& &\mathrm{quartered}&\quad\mathbf{48 }&& 58 &&\mathrm{Torgerson1933} \\
&\mathrm{Cauliflower}& \mathrm{florets}&\quad 6 &&\mathbf{ 5}&&\mathrm{Warthesen1984} \\
& &\mathrm{ with 2.5 cm stem}&\quad\mathbf{10 }&& 13 &&\mathrm{Pellegrini2010} \\
&\mathrm{Chard}& &\quad\mathbf{ 5 }&& 10 &&\mathrm{Lee2018} \\
&\mathrm{Carrots}& \mathrm{sliced}&\quad 10 &&\mathbf{ 7}&&\mathrm{Rennie2010} \\
& &\mathrm{2.5\;cm\;cylinder}&\quad\mathbf{ 8 }&& 10 &&\mathrm{TorresdeCastro2020} \\
& &\sim\mathrm{2.5\;cm\;cylinder}&\quad\mathbf{10 }&&\mathbf{10}&&\mathrm{Warthesen1984} \\
& &\mathrm{2\;cm\;cube}&\quad\mathbf{12 }&& 15 &&\mathrm{Lee2018} \\
& &\mathrm{2.5\;cm\;cube}&\quad\mathbf{30 }&& 35 &&\mathrm{Torgerson1933} \\
&\mathrm{Corn\ (sweet)}& &\quad\mathbf{ 5 }&& 20 &&\mathrm{Zhang2022} \\
&\mathrm{Crayfish}& &\quad\mathbf{ 4 }&&\mathbf{ 4}&&\mathrm{Li2023} \\
&\mathrm{Crown\ Daisy}& &\quad\mathbf{ 5 }&& 10 &&\mathrm{Lee2018} \\
&\mathrm{Fish\ Filets\ (Sea\ Bass)}& &\quad\mathbf{10 }&&\mathbf{10}&&\mathrm{NievaEchevarría2017} \\
&\mathrm{Mallow}& &\quad\mathbf{ 5 }&& 10 &&\mathrm{Lee2018} \\
&\mathrm{Noodles\ (wheat)}& &\quad\mathbf{ 3.5}&& 15 &&\mathrm{Tian2020} \\
&\mathrm{Pak\;Choi}& &\quad\mathbf{10 }&& 15 &&\mathrm{Chen2019} \\
&\mathrm{Peas}& &\quad\mathbf{10 }&& 8 &&\mathrm{Warthesen1984} \\
&\mathrm{Perilla\ Leaf}& &\quad\mathbf{ 5 }&& 10 &&\mathrm{Lee2018} \\
&\mathrm{Pork} &\mathrm{Ham}&\quad\mathbf{27 }&&\mathbf{30}&&\mathrm{Jeon2013} \\
& &\mathrm{Sausage}&\quad\mathbf{10 }&& 19 &&\mathrm{Prapasuwannakul2017} \\
&\mathrm{Potatoes}&\mathrm{2\,cm\;cube}&\quad\mathbf{20 }&&\mathbf{20}&&\mathrm{Lee2018} \\
& &\mathrm{\sim2.5\,cm\;cube}&\quad\mathbf{20 }&& 32 &&\mathrm{Torgerson1933} \\
& &\mathrm{\sim6.5\,cm\;diameter}&\quad\mathbf{28 }&& 40 &&\mathrm{Warthesen1984} \\
&\mathrm{Semolina}& &\quad\mathbf{ 5 }&& 10 &&\mathrm{ElYamlahi2014} \\
&\mathrm{Spinach}& &\quad\mathbf{ 4 }&& 9 &&\mathrm{Warthesen1984} \\
&& &\quad\mathbf{ 5 }&& 10 &&\mathrm{Lee2018} \\
&& &\quad\mathbf{ 8 }&& 10 &&\mathrm{Caparrotta2019} \\
&\mathrm{Squid}& &\quad\mathbf{ 5 }&&\mathbf{ 5}&&\mathrm{Xiao2021} \\
&\mathrm{Sweet\;Potato}& &\quad\mathbf{20 }&&\mathbf{20}&&\mathrm{Lee2018} \\
&\mathrm{Zucchini}& &\quad 6 &&\mathbf{ 4}&&\mathrm{Warthesen1984} \\
&& &\quad\mathbf{10 }&& 12 &&\mathrm{TorresdeCastro2020} \\
&& &\quad\mathbf{12 }&& 15 &&\mathrm{Lee2018} \\
\end{align}
$$
Links to Sources (Hovering over link shows full citation):
Bongoni2014,
Caparrotta2019,
Chen2019,
DanowskaOziewicz2020,
ElYamlahi2014,
Hazard2010,
Jeon2013,
Lee2018,
Li2023,
NievaEchevarría2017,
Pellegrini2010,
Prapasuwannakul2017,
Rennie2010,
Tian2020,
Torgerson1933, TorresDeCastro2020,
Xiao2021,
Warthesen1984,
Zhang2022.
The average cooking time used for steaming is a third longer than the average time for boiling.
Another way to compare steaming and boiling is to measure how fast a vegetable softens. As shown in the plot below, Buratti et al. (2020) found that the desired firmness was reached faster by boiling than steaming for cauliflowers, carrots, and sweet potatoes.
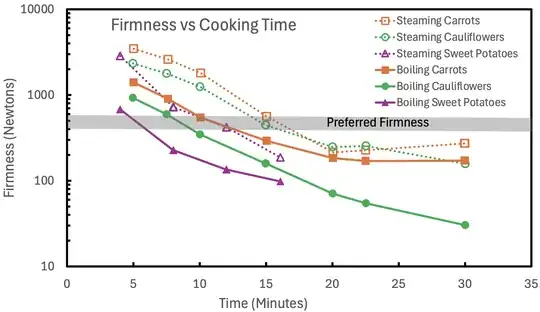
In addition to the data showing that steaming is usually slower than boiling, the often small differences between steaming and boiling times indicate that the limiting factor on cooking times is not the heat transfer from the water/steam through the surface of the food item, but the effective thermal conductivity within the food.
Heat transfer coefficients are not the whole story
The nice answer by @Testina is certainly correct about steam being able to transfer more heat per gram of water, but as @Sophie-Swett's comment noted, this doesn't mean that that rate of heat transfer into the food is faster.
It is true, as @Eric-Tower's useful comment noted, according to steam experts at tlv.com the convective heat transfer coefficient for steam ($h \approx 6000 – 15000 \,\mathrm{W/m^2/K}$) is much higher than that of boiling water ($h \approx 1000\,\mathrm{W/m^2/K}$). This does not, however, necessarily translate into a much greater overall heat transfer coefficient ($U$) into an object, which also depends on the length scale ($L$) and thermal conductivity ($\lambda$) of the object:
$$\frac{1}{U}\sim \frac{1}{h}+\frac{L}{\lambda}$$
In the examples given in the cited source, steam heating is only 17% faster than boiling water at heating a steel kettle, and adding just a 1 mm layer of glass to the outside of the kettle reduces steam's advantage to just 9%.
The thermal conductivity of food is even less than glass and food items are typically thicker than 1 mm, so any theoretical advantage of steam heat penetrating into food may often be negligible in practice. For example, for a 3.5 cm radius spherical potato with thermal conductivity of $0.6\,\mathrm{W/m/K}$ we expect:
$$\begin{align}
U_{boiling}&\sim \left(\frac{1}{1000\,\mathrm{W/m^2/K}}+\frac{0.035\,\mathrm{m}}{0.6\,\mathrm{W/m/K}}\right)^{-1}=16.85\,\mathrm{W/m^2/K}\\
U_{steaming}&\sim \left(\frac{1}{100\,\mathrm{W/m^2/K}}+\frac{0.035\,\mathrm{m}}{0.6\,\mathrm{W/m/K}}\right)^{-1}=17.11\,\mathrm{W/m^2/K}
\end{align}$$
So the theoretical advantage of steaming over boiling for our potato is less than $2$%, but perhaps the more important point is that we expect cooking times for boiling and steaming to be very similar. Even for smaller food items, the advantage of steam should be less than $10$%. The observed slight advantage of boiling over steaming is presumably due to minor effects which we haven't considered, e.g. liquid vs gas porosity of the food item, possibly lower effective temperature of the steam+air, ….
Nevertheless, "Introduction to thermal food processes by steam and hot water" did report that steam is more common than boiling for industrial food processing:
Because of its advantages, saturated steam is the most often utilized heating medium for commercial sterilization of packaged goods. When saturated vapor condenses on the container's surface, latent heat is transmitted to the food.
Because the heat transmission coefficient of condensing steam is larger than that of hot water, steam blanching takes less time than water blanching for sliced and small products. Larger products or parts of product, on the other hand, can be “overblanched” near the surface and “underblanched” at the center due to strong temperature gradients between the surface and the center of the product.
The advantage of steam in industrial processing, however, may have less to do with any better heat transmission than with the fact the steam is 3 orders of magnitude less dense than boiling water. It is easier to move steam than water, and easier to move product through steam than through water on a production line.
Human Factors Affecting Cooking Times
As emphasized by the well-known saying that "A watched pot never boils", waiting for water to boil can stretch out time in our minds, so it may also be more psychology than physics that makes us not notice that boiling water actually heats food faster than steam.
Steaming may also often be faster in part because of many people have different expectations for steamed and boiled food.
If you expect steamed vegetables to be firm and boiled vegetables to be soft, you would naturally use shorter times for steaming than boiling.
This might help explain the wide variations in recommended cooking times from different sources.
For example, Betty Crocker recommends longer steaming times than boiling times for most vegetables, this vegetable cooking cheat sheet usually recommends shorter steaming than boiling times, and this site is about evenly split.
Related Questions
