Suppose I had a piece of glass (just as an example) at room temperature, let's say T0=293 K, and I moved it into a dark room that was TR=1 K. I assume the glass will radiate heat until it and the room are the same temperature.
What does its thermal spectrum look like during that process?
You have correctly noted that we are dealing here with a process, that is development in time - and the radiation spectrum would change over time.
A body in thermal equilibrium with its environment has emission spectrum close to black body radiation. It is not exactly black body radiation, since no object absorbs all the radiation incident on it, but black body is often a good approximation.
A large (macroscopic), object not in equilibrium with its environment also emits a spectrum close to black body spectrum - the reason for that is that the radiation emitted is only a small part of radiation trapped inside the object, which is in equilibrium with it, i.e., it is black body radiation as well. (This allows us to determine temperature of stars... while the deviations from the black body spectrum allow us top determine their chemical composition.)
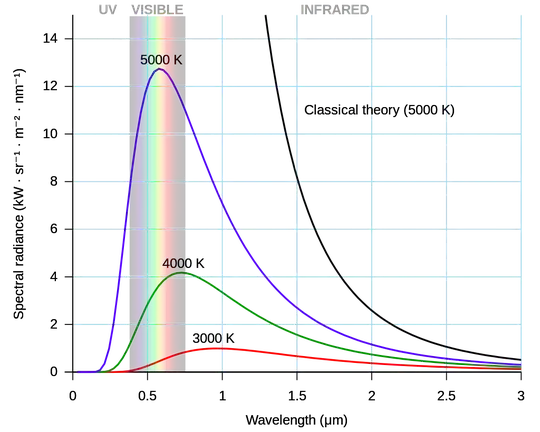
Thus, the spectrum will remain nearly black body throughout the cooling process - provided that the cooling is slow, which is usually the case, when it is cooling via contact with air, a poor heat conductor. The shape of the spectrum will gradually evolve with the temperature, which is the parameter determining the shape of the black body curve:
Remark
I don't know, if there are any specific caveats related to the type of the material chosen in this question (a piece of glass), so I apologize, if thsi answer is too generic.
Related
Equilibrium in blackbody radiation
How does radiation become black-body radiation?
