GILBERT LEWIS model of the atomic structure and a few unusual suggestions
Niels Bohr: „Thus LEWIS, who in several respects independently came to the same conclusions as Kossel, suggested that the number 8 characterizing the first groups of the periodic system might indicate a constitution of the outer atomic groups where the electrons within each group formed a configuration like the corners of a cube.“(1) (p.112) . . . (Valence theory from Gilbert N. Lewis)
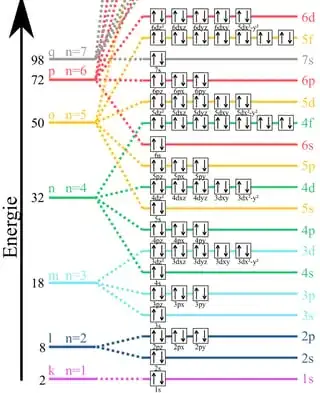
On the basis of the first two periods, Bohr assumes the formula $2n^2$ for all orbitals. This leads to this orbital division:
Presumption
Langmuir's shell model with 2, 8, 8, 18, 18, ... electrons per shell follows the empirically established periodic table of the elements.
Bohr's shell model according to the formula with 2, 8, 18, 32, ... electrons is not comprehensible.
And Bohr continues: „It is to be remarked, however, that such a “static” model of electronic configuration will not be possible if we assume the forces within the atom to be due exclusively to the electric charges of the particles.“(1) (p.112)
If we assume for a moment that when electrons are captured in the atom, the electric field of the electrons is reduced by the emission of EM radiation, we could follow Lewis. The magnetic dipole properties of the electron would be the starting point for the arrangement of the electrons in the shells. All we need is the willingness to think about it,
- that we have only ever measured the standar charge of the electron for free electrons
- that the emission of EM radiation could be fed by the fields of the electron (and proton)
- that the Pauli exclusion principle has its cause precisely in the pairwise interaction of the magnetic dipoles of the electrons.
“LANGMUIR, who has attempted … to account not only for the occurrence of the first octaves, but also for the longer periods of the periodic system, supposes therefore the structure of the atoms to be governed by forces whose nature is unknown to us.“ (1) (p.112-113)
The magnetic moment (electrons are magnetic dipoles, which is all too often regarded as unimportant) is precisely the force that was not yet known in Langmuir's time or was later regarded as unworthy of consideration.
„He conceives the atom to possess a “cellular structure,” so that each electron is in advance assigned a place in a cell and these cells are arranged in shells in such a manner, that the various shells from the nucleus of the atom outward contain exactly the same number of places as the periods in the periodic system proceeding in the direction of increasing atomic number.“ (1) (p.113)
For me, the best argument in favor of Lewis's model is the phenomenon of $sp^3$ hybridization, for the explanation of which QM has to stand on its head, although this arises quite naturally in the cubic atomic model.
And if there has to be a calculation based on the spherical harmonics, then please do not assume a Carthean symmetry as the basis, but rather one based on the thetrahedron with $l=3, m=2, l-m=1$.
(1) The Theory of Spectra and Atomic Constitution
THREE ESSAYS by BY NIELS BOHR
CAMBRIDGE AT THE UNIVERSITY PRESS 1922
