The system is not at equilibrium, so there is energy flowing between the various parts of it (and also energy flowing into and out of the system as a whole).
But thermal energy still flows only from warmer bodies to colder bodies. Even in a non-equilibrium state, one object cannot be hotter than the things it is exchanging thermal energy with. And the rate at which thermal energy flows between objects depends on the difference in temperature between them (with greater difference leading to faster transfer, from the hotter object to the colder). That is enough to deduce an answer to your question.
There is a lot of thermal energy coming from the flame, which is much hotter than 100° C. The pot is much colder than the flame, so thermal energy flows into the pot.
Thermal energy also flows out of the pot, into the surrounding air and into the water. We'll imagine that we have an essentially infinite reservoir of cool air that can easily carry away the heat, but won't worry about the exact temperature; if it's liveable for the human operating the stove it's definitely much less than 100°! So the surrounding air remains at a lower temperature than the pot and energy will continue to flow out of the pot into the air.
Energy is also flowing from the pot into the water. We know it must because energy is also leaving the water into the air (both by direct contact and in the form of energy carried away by the water that evaporates into steam). If thermal energy were not flowing into the water from the pot, then the water would be cooling down and would not remain boiling at 100° C.
So the pot must be at least a bit hotter than 100°. If it were cooler then it would actually be taking thermal energy from the 100° water instead of supplying energy to it, and if it were exactly 100° then the energy flow between the pot and the water would be zero. In either case the water would cool down until its temperature were low enough below the pot that the rate of energy transfer from the pot to the water is enough to replace the energy the water is losing to the air, rather than remaining boiling. So if we've reached a steady boil, the pot is hotter than 100°. (You can see this by boiling water in a kettle, then turning on the heat under a cold pot and pouring the boiling water in; the water will stop boiling as it first loses energy heating up the cold pot, and only returns to the boil once the gas flame has heated the pot sufficiently)
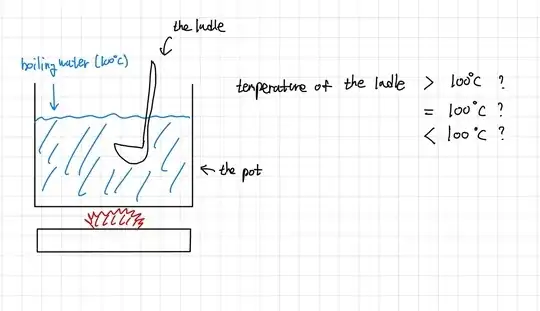
Finally consider the ladle. As you've drawn it, it is in contact with the water and with the surrounding air. So it cannot reach a temperature hotter than both of them; if it were it would be losing heat to its surroundings. At a steady state (where things will remain roughly the same until we alter the gas setting or too much water evaporates), the average temperature of the ladle must be near the temperature of the water, but a little below. The air is definitely colder, so the exposed part of the ladle will be transferring heat energy to the air. For it to remain a roughly steady average temperature, the rate of energy flow from the water into the ladle must be equal to the rate of energy flow from the ladle to the cooler air. So it must be below 100° or there would be no energy flowing from the water to the ladle to replace the energy lost to the air. The exact average temperature this will be depends on how fast heat can conduct from the underwater parts of the ladle to the exposed handle (it's not actually all one temperature), what the ladle is made of, the exact temperature and composition of the air, etc etc. But we can see that these two flows have to be equal and opposite, or we wouldn't have a steady state yet.
If the ladle were cooler than this steady state temperature (as when you first put it in the water), then the difference in temperature from the 100° water would be larger and the underwater part would heat up faster than the exposed part can lose energy to the air (indeed if the ladle is colder than the air, the air-exposed handle will be heating it up too!). So it will heat up, reducing the temperature difference with the water and the energy flow, until those two flows are in balance and we do reach that steady state.
If the ladle were hotter than the steady state temperature then it's losing energy to the air faster than it's gaining energy from the water, so it would be cooling down. So there's no mechanism that could even maintain a temperature hotter than the steady state temperature, let alone a mechanism that could allow it to reach a hotter temperature in the first place.
The feature of this system that allows it to reach a known pseudo-equilibrium (where things reach and hold a particular temperature for a while, even though lots of energy is flowing through the system and we don't even know exactly how much energy is coming from the flame) is the boiling point of water. If the water didn't evaporate then it would just keep heating up above 100°. So the pot wouldn't stop heating up when the flame's heating effect is equal to the cooling effect of 100° water; the water's temperature would rise and the cooling effect would be less so the flame could heat the pot up more, and so on.
Eventually there would be another pseudo-equilibrium point where the flame just isn't putting out enough energy to raise the temperature of the pot (and indirectly the water) any higher, because of the cooling effect of the air (which we're presuming can be infinitely replaced and so never actually heat up). But the exact state would depend on how much energy is coming from the flame; what setting we have the gas at. With water boiling at a fixed temperature of 100°, that puts the contents of the pot in a known state (although the temperature of the pot itself will be at whatever temperature balances the energy it transfers to 100° water with the energy it receives from the flame; we don't know what that is). If the water is receiving more or less energy it will evaporate faster or slower, but the energy in the evaporated water is carried away by the air. The evaporation makes the system self-adjusting to a wide range of energy input rates from the flame.
This is exactly why cooking things in boiling water is such an easy and important technique humans have been using for many thousands of years! As long as you can get your cooking heat source hot enough to boil water (and within a reasonable upper limit that's hard to reach accidentally), it doesn't matter very much exactly how hot it is. The boiling water will have a consistent effect on the food you're cooking every time! And because the water is receiving "too much" energy to remain at 100° but having the excess carried away as steam, the system can often self-adjust when you drop some colder food into the water to start cooking; as it's now losing more energy heating up the food it simply has less excess going to evaporation (unless you put in so much food that is so much colder that the water loses heat to it faster than it can pull heat from the pot; I see this when I cook frozen vegetables in boiling water). If the 100° temperature was being maintained by a very careful balancing of input and output energy rater than the boiling point effect, then any change at all would affect the balance and change the cooking temperature quite a bit, requiring a lot more fine tuning and control from the cook.