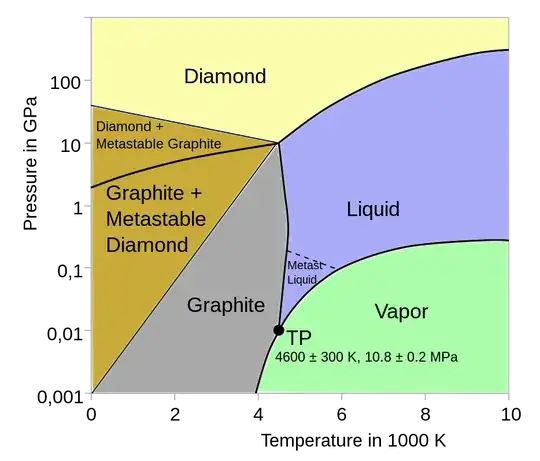
I'm a litte confused by this diagram. What does the "Diamond + metastasble Graphite" and "Graphite + metastable Diamond" regions mean? I mean at room temperature and Pressures, carbon is in the Graphite region, and yet diamonds don't normally turn into Graphite. So what's the difference between the Graphite region and the "Graphite + metastable Diamond" region?
Asked
Active
Viewed 518 times
1 Answers
4
I would say that it is a qualitative "effective" phase diagram showing not only the thermodynamic stable phases but the possible presence and coexistence of metastable phases with strong kinetic stabilization (thus stable over macroscopic times).
I call it qualitative because a one-component system cannot have a coexistence of more than three stable phases (according to Gibb's phase rule, which is a consequence of the general coexistence conditions). The presence of a point where apparently five phases coexist means that the graphic resolution around that point is poor, and more careful scrutiny should show the presence of separate but close triple points.
GiorgioP-DoomsdayClockIsAt-89
- 39,465
- 9
- 53
- 119