Motivated by question Can IC engines be modeled as Carnot engines?. I am wondering whether/how Carnot theorem could be generalized to other kinds of devices performing "useful work", such as, e.g.:
- Motor (or generator) fed by a battery
- Nuclear power generators
- Solar cells
- Water wheels
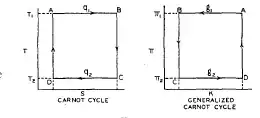
I think that the theorem must be generalized in at least three ways:
- Operating media is neither a gas nor a liquid - that is the reasoning based on isothermic adiabatic expansions might not apply.
- Generalizing the concept of temperature (introducing "effective temperature"?) - e.g., in case of a batter or a water wheel, we do not have two reservoirs with different temperatures to properly speak of, but rather two reservoirs with different (chemical) potential.
- Generalizing the concept of useful work - solar cell and water wheel are not really transferring the energy between two reservoirs - the energy already flows, and the device simply diverts a part of this energy into work. But, since the energy flows anyway, it is not clear whether/how the part of it that is diverted is useful: e.g., how is the current generated by a solar cell is more useful than the heat generated in the surface illuminated (which may be also "useful" in everyday sense.)
Perhaps, there is not much left of Carnot theorem with all these generalizations, and we simply need to consider it as limited to a particular class of phenomena? If so, are there other upper boundaries on converting energy to work (that would be applicable to the devices cited in the beginning?)