As far as I know, CO$_2$ and O$_2$ both are almost perfect ideal gas at atmospheric conditions, so the volume they occupy shouldn't affect each other.
Room temperature CO$_2$ and O$_2$ can often be treated as ideal gases, but this is not one of those times. The issue is collisions. The mean free path of an air molecule (e.g. O$_2$) at STP is about 60 nm - an incredibly small distance. If you divided a container in half using a partition and filled one side with O$_2$ and the other side with CO$_2$, it would take an extremely long time (apparently on the order of 10 minutes) for the gases to mix completely once the partition was removed.
As a result, your CO$_2$ will tend to move as a fairly cohesive mass of fluid on the scale of seconds, which is the time scale you're studying when you dump it out onto a candle. This is also why we talk about air parcels and cold fronts in meteorology - distinct "packets" of air undergoing bulk motion tend to move around each other rather than mix together, because the time required for the latter is far longer than the time required for the former.
So really, the remarkable thing is not that ideal gas approximation fails, but rather that it so often succeeds. The key insight is that collisions cause a gas to relax toward equilibrium. Once that equilibrium is achieved, collisions no longer alter the macroscopic properties of the gas, and so as long as there are no appreciable long-range interactions between gas particles, the ideal gas approximation tends to yield the correct results. As soon as the gas is no longer in equilibrium - say, when the CO$_2$ is poured out of its container - the approximation fails badly until collisions have caused the system to equilibriate again.
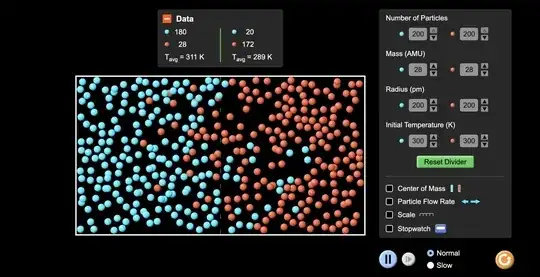
This excellent simulation from PhET illustrates precisely this phenomenon, so it's worth a look.