When I pick up my scuba tanks from the dive shop, I measure the nitrogen and oxygen levels in it. I check that they are close to what I specified and calculate the maximum depth it's safe to go to to ensure acute oxygen toxicity is not an issue. I also need to know the gas mix so that when I do the dive I can accurately calculate my decompression obligations (or try to avoid having any at all.) One is taught to not measure the mix too soon after the tank is filled to ensure they have mixed thoroughly.
When I was a kid I was taught that the temperature of a gas was a measure of the average speed of the molecules and that the speeds of the molecules in the gas would be normally distributed about the mean speed, some moving very slowly and some very fast. I had always imagined the average speed to be quite zippy and that a mix of gasses would be pretty evenly mixed quite quickly.
According to this answer How fast do molecules move in objects? the molecules in a gas at 300 K, roughly the temperature on a hot day, the molecules move at a speed of about 300 m/s.
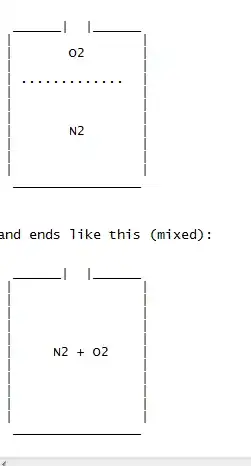
So my question is, if one added to 8 litres of nitrogen 2 litres of oxygen, how quickly would the mixture be thoroughly mixed? At least mixed enough that any measurement would be the same to within .1 % throughout the cylinder. (That's a tenth of one percent.)
So the cylinder starts like this (unmixed)
I have no idea how to calculate this.