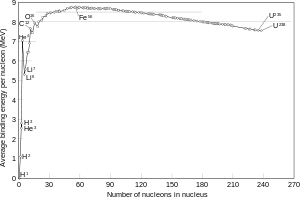
The nuclear binding energy, is the energy that is needed to seperate the nucleons in a nucleus. And binding energy is also defined as the energy given out when a nucleus forms from nucleons.
Also the larger the nucleus is, the more energy is required to break it apart, so why doesn't that mean that larger nuclei are more stable? I mean Uranium has a lower binding energy per nucleon than Iron, but there are many many more nucleons in Uranium that Iron so the total binding energy is going to be much greater.
Basically I don't understand why whether an element gives out energy by fusion or fission (why the lighter element provide energy by fusion not fission and vice versa for heavy elements) depends on binding energy per nucleon and not "total" binding energy