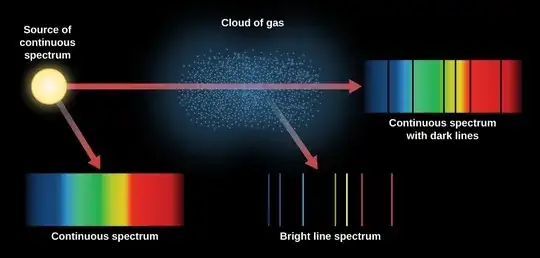
lately I have come across the hydrogen line spectrum, I quite understand it as I could easily solve all numerical questions. However, conceptually I feel stuck at two points.
If an electron absorbs the em radiation of a particular wavelength to jump to a higher shell say $n=1$ to $n=3$, then when it returns to the ground state (from $n=3$ to $n=1$) will it emit the radiation of the same wavelength or of a different wavelength. Kindly explain what exactly happens?
In the absorption spectrum of hydrogen, em wavelengths of 434 nm, 486 nm, and 656 nm are absorbed because they correspond to the energy difference between the shells so they are not present on the photographic plate but I don't understand that if excited electrons (from the absorbed light) jump back to their ground state in nanoseconds releasing the same radiation with the same wavelength then why does it not appear in the spectrum? Also, how do the electrons figure out which light to be absorbed (I know it sounds dumb but I just can't understand how do electrons figure out that a particular wavelength does not suffice the energy requirement to jump?)
P.S.: Please consider my doubt and excuse it if they appear dumb as I have taken these questions to various platforms and have never received any satisfactory response.