Different bits of the fire have different characteristics, the spectrum of a flame would usually consist of discrete line radiation perhaps superposed on a weaker continuum. However, the base of the fire, especially say in the cavities between any burning material would emit radiation that more closely approximates the Planck function. In this respect, a just-lit fire would have light dominated by "flame" and the spectrum would not be Planckian, but a well-established hot fire with a lot of radiation coming from its "heart" would be more blackbody-like.
The flames that you see would usually be "optically thin" - that is they are transparent to their own and other radiation. In such circumstances, the flames emit light that usually correspond to discrete transitions at particular wavelengths - about as dissimilar to the Planck function as it could be. This is how "flame tests" for the presence of different chemical elements work. If the flame was hot enough to ionise atoms you would also get some recombination continuum radiation, but as I said, most flames are optically thin, so the radiation never gets the chance to come into equilibrium with the matter at some particular temperature and you would not get the Planck spectrum. If you can see through the flame, at any wavelength, then it isn't radiating a Planck spectrum.
On the other hand, the space between the coals of a hot fire can provide a reasonable simulacrum to the cavity radiation of an ideal blackbody. Here, we are looking into an enclosed space where the radiation field has had a chance to come into equilibrium with the surrounding material. This is still just an approximation - but a clue that you are looking at something close to the Planck spectrum comes when you start to lose the ability to discern the texture or shape of the material within the cavity. That is because everything is at a similar temperature and the radiation field is starting to approach isotropy.
The above discussion by the way is often why one will notice that it isn't the flames of a fire that give off the most heat, it is the base of the fire. That is because blackbody radiation is the most efficient way that a thermal radiator can lose heat (radiatively).
EDIT:
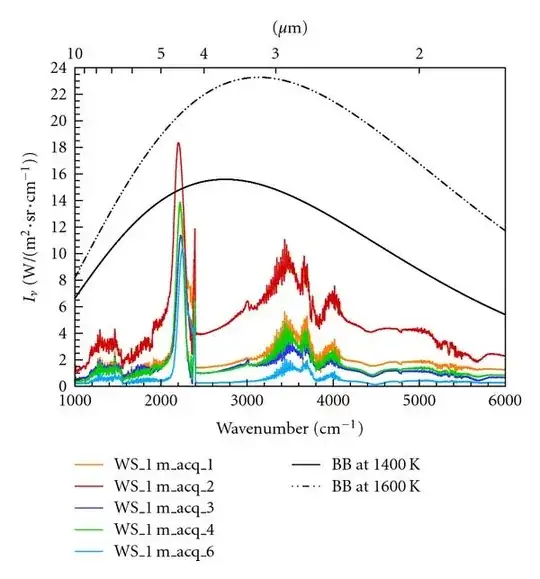
Here are some spectra taken of flames from a simulated wildfire, compared with blackbody spectra - not very Planckian (from Boulet et al. 2011). The main peak at 2300 cm$^{-1}$ is due to radiation from CO$_2$ and CO molecules. The conclusion in this paper is that the temperature is about 1500K and the CO$_2$/CO radiation is optically thick and thus reaches the Planck function, but that the flames are optically thin at all other wavelengths.