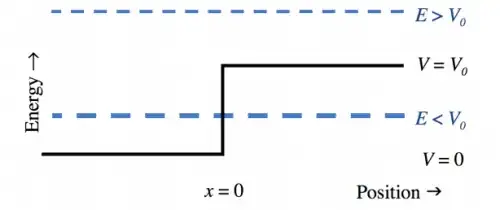
When an electron with E < V approaches a step in potential energy, the wave function will exponentially decay at the step, meaning there is still a finite possibility of the electron being found at x > 0:
My question: presumably the electron can't be found "inside" the step? So if it is found at X > 0, would it be found with E > V (i.e. above the step)? Or does such tunneling only have a meaning if the decaying wavefunction can reach the other side of the step?