I wanna consider some cases and make sure if im right ,
if i have fixed volume balloon filled with helium , and let balloon fly in air ,
1)
if air temperature increases , ( without increasing temp of helium ) that will decrease density of air , so buoyancy force decreases.
2)
but if helium temperature increase , volume is constant so pressure increases then helium density is constant , and density of air decreases so the buoyancy force decreases .
3)
but if volume of helium increases with constant pressure , helium density decreases , air density decreases so no effect .
So buoyancy force for immersed body contained contained fluid decreases with increasing temperature if temperature of internal fluid is insulated or constant volume , buoyancy force will not change with changing temp if volume change .
[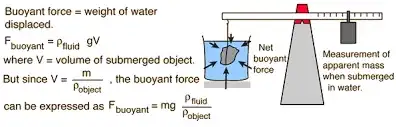 ]
]
ive read this answer here
Why do helium balloons rise and fall?
but i see different cases when volume of helium constant , as air density decreases so buoyancy force will decrease