There are a lot of questions about this topic on this site, none of them answer my question specifically.
I have read this question:
What does a photon emitted by an atom "look" like?
I think of the emitted photon as a point particle (but with a polarization vector) traveling in a straight line from the atom to the measuring device.
Photon description of quantum-optical interference experiments
For some people, a photon is a dimensionless point traveling on a world line (Eugene Wigner's definition of a particle).
Shooting a single photon through a double slit
The photons do not have a well defined trajectory. The diagram shows them as if they were little balls travelling along a well defined path, however the photons are delocalised and don't have a specific position or direction of motion.
How come lenses alter the path of photons?
because photons take all paths, but since their underlying physics is "wavey" different paths can "interfere.
How do single photons travel from here to there
This calculation assumes that light simultaneously travels over all possible paths. To what extent this is just a calculational device and to what extent it reflects an underlying physical reality is a matter of opinion.
Can photons travel faster than $c$? (Feynman Lectures)
Indeed, nothing in nature moves around on all possible paths, in reality it's a field that permeates the vacuum which has quantized solutions.
There are mainly two thoughts:
the photon travels in a straight line, and that explains why it only interacts with a certain atom, meaning, that the photon can only be detected once, and the photon will not interact with the other atoms because they are out of its trajectory
the photon takes all paths, and that is why it truly goes through all possible avenues, explaining experiments like interference
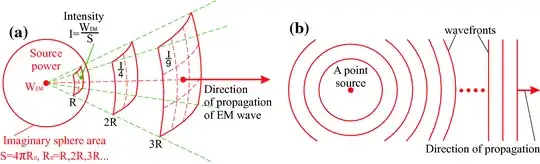
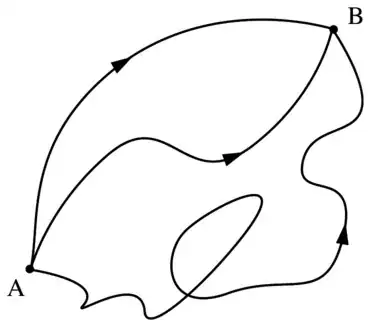
Now these lead to two different propagation pictures. On the top, you can see the photon (EM wavepacket) spreading spherically in all directions. On the bottom, you can see a photon traveling from A to B, taking all possible paths.
But which picture could be experimentally proven to be correct? The picture on the top cannot explain why the photon misses all the other atoms, that is, why it is not interacting with other atoms, because all atoms are in its way basically (it spreads spherically). The picture on the bottom cannot explain diffraction, because the photons is shown not to spread like a wave (cannot interfere), but just like a billiard ball on different paths.
After the question was closed, I am editing to clarify (to reopen), that (as I understand), the question was closed due to the word "really" and what it physically means for the photon to take all paths, or whether the picture on the top (spherical spread) physically describes what is happening. I now revise these words, and the "physically" is meant here to say "experimentally provable".
Question:
- Do photons take all paths or not?