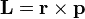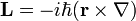I read this on Wikipedia:
[...] That most tangible way of expressing the essence of quantum mechanics is that we live in a universe of quantized angular momentum and the Planck constant is the quantum. [...]
And I'm wondering if anyone can explain this a little more. Is it saying that ALL action is made up of angular momentum which is quantised in units of Planck's constant? Or is it just saying that all matter is composed of particles which have quantised angular momentum?
If the answer is the prior, this would mean that a photon's movement through space is as a result of angular momentum, which seems strange.
Anyway, if you could help me out with a little explanation, that would be great.