In Freeman Dyson's classic 1979 Time Without End paper he points out that, if proton decay does not occur, then normal matter will spontaneously fuse to iron on a timescale of $10^{1500}$ years, and heavier elements will undergo fission or alpha emission, again producing iron (although he does not calculate the timescale). My question is how correct this picture is.
It is a well-known quibble that strictly speaking, $^{56}$Fe is not the most tightly bound nucleus: it has 8790.4 keV binding energy per particle but $^{58}$Fe has 8792.3 and $^{62}$Ni has 8794.5. More subtly, this depends on whether we consider adding the right number of nucleons or the right number of protons and neutrons. So it might appear that the long-term composition of matter would hence be nickel-dominated.
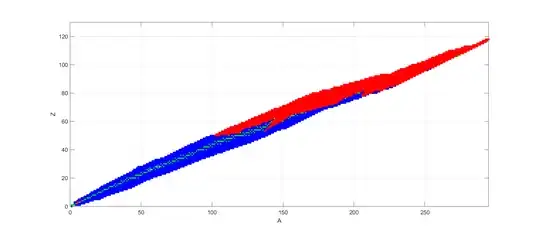
However, just like the stellar formation processes favour $^{56}$Fe over $^{62}$Ni despite the energy difference because of the relative scarcity of neutrons to bridge the gap between them, the actual decay processes in the far future also seem to lead to trapping in other isotopes. $\alpha$-decays require a positive Q-value for $^{A}_{Z}X\rightarrow ^{A-4}_{Z-2}Y+\alpha+Q$: this becomes common above $A>102$, but below the energy difference is too small to allow $\alpha$-decay. $\beta$-decay to the rescue! They (and electron capture) happen across the entire $A$ range, but only change $Z$ by $\pm1$. So this appears to lead to a lock-in where heavy element decay get stuck in the region $62>A>102$. In this argument I have assumed that any energetically allowed transition will eventually happen; at least some $\beta$-decays are presumably blocked by spin considerations.
Plot of hypothetical decays in (A,Z) plane: blue beta decay/electron capture, red alpha decays. Green isotopes do not have any energetically allowed decays. White circles mark iron-56 and nickel-62.
I seriously doubt Dyson would have made a mistake (even though this is an extremely minor part of the paper), so what is going on? Spontaneous fission reducing everything to the iron peak? Or is the long-term chemical composition of the universe a mixture of stable heavyish elements?
[ Some other minor issues: in $\Lambda$CDM cosmology rapid expansion also makes many atoms and molecules isolated so their fusion processes end long before iron. There are also issues with spontaneous ionization and possibly nuclear decay due to the Herzfeld "paradox" of divergent partition functions for completely isolated atoms. And, as Dyson pointed out, iron is metastable to tunnelling into neutron star or black hole states. ]