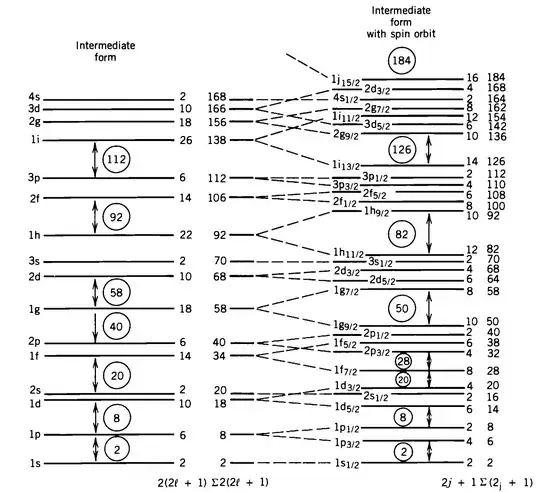
In atomic physics, the spin-orbit is a small correction between 1/1000 and 10ppm, so fairly small. In contrast, in nuclear physics the inclusion of the spin-orbit interaction is necessary to reproduce the basic level scheme, see picture below (from Kenneth Krane, Introductory Nuclear physics). Why is that?
I imagine it has to do with the $r$-dependence: In a semi-classical calculation, the energy correction due to the spin-orbit interaction has a $1/r^3$ dependency. However, I have only seen this calculation for the atomic case and not for the nuclear one.