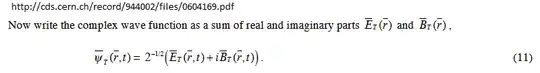How can the energy of a photon be calculated with the frequency of light if light is not considered as a wave when it is considered as a particle?
2 Answers
The present standard model of particle physics has the photon as one of the basic particles. The mathematical theory is quantum electrodynamics.
Light is very well modeled with Maxwell's equations , the classical theory of light. The two though are connected mathematically. The photon is described by a wavefunction which is the solution of a quantized version of Maxwell's equations.
Quantization introduces the E and B fields in the complex wavefunction and is the way that the frequency observed in the classical light is connected with the energy of the photon at a mathematical level.
There exists a mathematical derivatiion of how the classical light emerges from the zillions of photons in quantum electrodynamics here . The classical light emerges from the superposition of the wavefunctions of the zillions of photons.
The classical theory is very successful in fitting light behavior and predicting new set ups in a clean way that it is not necessary to refer to the collective QED behavior.
- 236,935
The best answer perhaps is that We know experimentally that the ene gy of a photon is h*nu We know that the photon is to be described in a counter-intuitive way to account for the experiments. And no one knows why nature is behaving in this absurd way.