It looks a lot like the result of diffusion-limited aggregation or more specifically diffusion limited cluster aggregation:
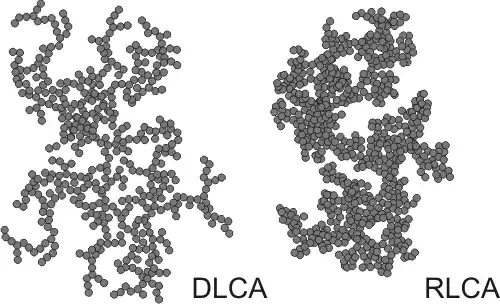
Image source.
If that's indeed the process responsible for the patterns, the question is then which particles are these. Likely candidates include dust and residues from previous use of the pot (milk, rice, etc.).
Edit: sammy gerbil's comment (and Ivan Neretin on the cross-posted Chemistry.SE question) suggest a chemical source for the particles: carbonates. The picture below (source) shows calcite particles aggregating into a lattice on the water surface of a stalactite drop.
Calcite precipitattion is well summarized in this Wikipedia entry, which describes (see also this thesis) how calcite growth is dominated by surface nucleation and coalescence and how (de)gassing of CO$_2$ from the water is a common process well known to lead to calcium carbonate precipitation.



