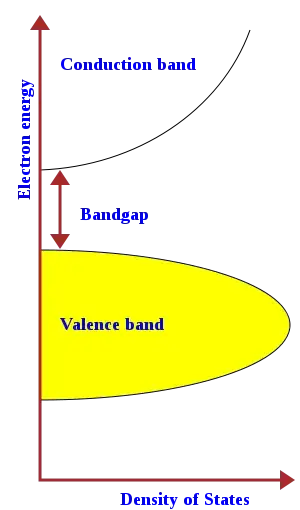
This is the classic picture of the band structure for an insulator (or semi-conductor):
The common explanation as to why this doesn't conduct is that the electrons fill the valence band perfectly full so that it is a traffic jam, and that there is not energy to excite electrons from the valence to the (empty) conduction band. The fermi energy level is somewhere in the bandgap.
However, why would we have just the right amount of electrons to fill the valence band but no more? This seems like too much of a coincidence given that insulating crystals are very common materials.
Supposed we doped the material to increase the charge carriers slightly. It appears that some electrons would be forced into the conduction band. But a small amount of doping doesn't make i.e. diamond a conductor (not even a poor conductor).
Perhaps the band structure "adjusts itself" (through electron interactions, etc) to perfectly fill the valence band? Or am I interpreting the diagrams incorrectly?