If you insist on thinking of photons as waves (which is fine as long as you ignore absorption, though you should really think of it as a disturbance in an electromagnetic field), you can more or less think of all of their amplitudes as being equal, and this is why your premise doesn't make sense.
More precisely, the amplitude of a single photon isn't constant in space and time, but in a broad sense the "total amplitude" of all photons is the same, which I explain more below. It is important to remember that they are not some infinitely extending wave (which would imply infinite energy) but rather localized probability density which is a wave packet. This is the wave dual of the particle.
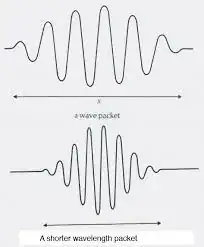
(A longer wave packet would have a smaller average amplitude, while a shorter one would have a greater amplitude, but the integral of the squared amplitude will always be 1)
And the amplitude of this wave does not even correspond to any observable quantity. In fact, according to schroedinger's equation, the amplitude of this wavefunction is not even a real number, but rather complex. However, the square of the amplitude IS something meaningful: probability density. When you are talking about individual photons (or any quantum particle), rather than thinking about energy intensity you should be thinking about the probability intensity of being here or there or being in a certain state. When you aggregate many photons together, the amplitudes of their superimposed probability densities would correspond in a sense to a real wave intensity on average, and that would be your connection to a classical idea of amplitude. But again, speaking of a single photon, an "intensity" doesn't really make sense.
The energy of a wave is proportional to the integral of the square of it's amplitude. (Note that it is not simply proportional to A^2. This would be the power, not the energy) This is why the energy of a wave packet of constant amplitude is proportional to the square of the amplitude. But this is not the case with a quantum wavefunction as it is a localized wave packet. Since this constant (the integral of probability of being anywhere in space) is always 1, the average or total square amplitude is constant for all photons so it does not appear in the equation for energy.
Comment on Ben cromwell's answer (sorry I can't comment):
@ben Crowell, while your answer is correct, I don't think it directly answers the OP's question. I want to point out that your second explanation is clearly the more relevant one and is at the core of what I was said in my answer. OP is starting with the concept that a photon can be thought of as a wave or wavefunction, which is true (although this comes with many caveats, the basic idea is true), and waves have amplitude. The key thing we agree on is that the amplitude is not constant over space or even for a given photon wavefunction as it depends on boundary conditions. This is exactly my point: since it must obey normalization, you can think (very roughly) of it's "total amplitude" as being constant and this is why it doesn't affect total energy.
