I've recently been seeing a lot of questions about why, when water is poured from a cup, some will dribble down the side. The best answer I've seen so far is that water is in a lower energy state when it adheres to a solid, but how exactly is it a lower energy state to cling to an overhang than, for example, falling straight down? Is there any explanation for this which a high school student might understand?
2 Answers
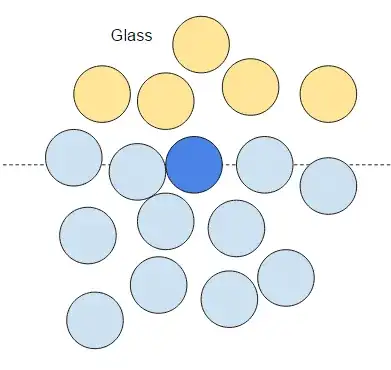
If you consider a water molecule somewhere in the bulk then it is surrounded on all sides by other water molecules. However a water molecule at the surface of the water is only partially surrounded by other water molecules:
So to get a water molecule to the surface you have in effect to pull away a number of the water molecules that originally surrounded it. But water molecules attract each other, which is why water is a liquid, so to pull away those other water molecules takes energy. The end result is that the energy of a water molecule is higher at the surface than in the bulk. This means there is an energy associated with the air-water interface, and the energy per unit area is equal to the surface tension.
Now suppose the water is in contact with something else. In the question you link that something else would be the surface of the glass:
Now the question is whether the water molecules attract or repel glass molecules. If water and glass molecules attract each other then the surface energy of the water-glass interface will be lower than water-air. However if water and glass molecules repel each other then the surface energy of the water-glass interface will be higher than water-air.
In fact the surface of glass is covered in OH groups that are much like the OH groups in water, so water and (clean) glass attract each other and the energy is lowered. That means the water will prefer to stick to the glass rather than peel off it because it lowers its energy by sticking to the glass.
However suppose we replace the glass by polyethylene, or we make the glass surface appear like polyethylene by covering it in an oily layer. In this case the water molecules and the surface of the glass will attract each other much less (there is always some small attraction due to Van der Waals forces). That makes it much easier to peel the water off the glass because the energy required is much smaller.
This is the basis of my answer to Why does water pouring from a glass sometimes travel down the side of the glass?. With clean glass the water tends to dribble because it wants to stick to the glass. With hydrophobed glass or plastic the water comes off the surface more easily so it is less likely to dribble.
- 367,598
While the other answers have discussed accurately the energy states of various positions of the water molecules in relation to their peers and the other fluids and solids adjacent, there is one additional factor that is responsible for the attraction as yet unmentioned. That is the polarity of the H2O molecule. Because the two hydrogen atoms reside more to one side of the oxygen, the field around the molecule is not uniform, being more positive on one side than the other. This makes the molecule "like" other polar molecules and conversely "dislike" non-polar ones. The glass molecules are quite polar as well, while the organic molecules discussed here are mostly not. So the water wants to stay together and also follow the surface of the glass if gravity does not say otherwise. While this experiment becomes rather comical on the ISS, the angle of the transition as well as its radius will be two factors to determine the success of your pour from the beaker or wine bottle.
- 1