So say you have a vertically polarized single photon impinging on an atom.
You cannot vertically polarize a photon. The photon has spin 1 which, because the mass of the photon is zero, will be either +1, i.e. in its direction of motion, or -1, against its direction of motion. The photon does not have an electric and magnetic field defining it so as to talk of classical polarization. Only momentum and $E=hν$
This can be seen pictorially in how photons build a polarized beam :
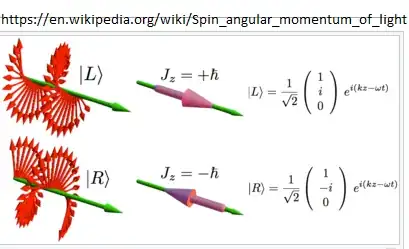
Note how the photon spin is + or - to its direction of motion, while the light has circular polarization.
The atom absorbs the photon and re-emits it. Does the re-emitted photon have the same polarization (vertical) as the incident photon? My gut tells me it does not, but I do not know.
Cannot be vertical for the individual photon, so the answer is no. If a photon with spin +1 is absorbed by an atom can give a photon of spin +1 to its direction of motion when it deexcites, will depend on the energy levels involved, i.e. the angular momenta for the individual energy levels and atoms.
Light, the quantum mechanical superposition of zillions of photons can have vertical (to its direction of motion) and circular polarization, depending how the individual wavefunctions of the photons $Ψ$ are added into one wavefunction for the ensemble. This link shows how the classical field is built up from the quantum mechanical fields.
