Raman spectroscopy essentially records photoluminescence:
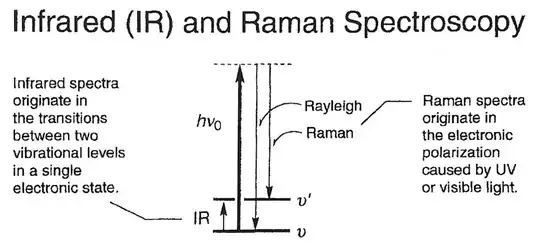 (source)
(source)
and a molecule is considered to be Raman active when there is a change of polarizability $\alpha$ (where $\mathbf{P}=\alpha \mathbf{E}$) during vibration, $\left(\frac{\partial \alpha}{\partial Q_k}\right)_0 \neq 0$:
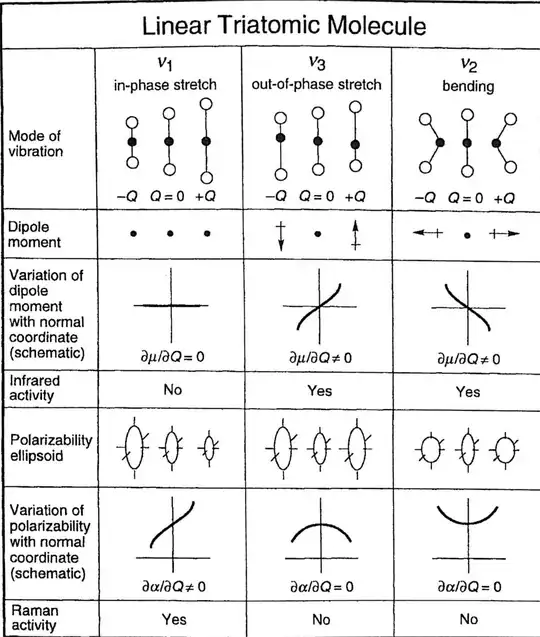 (source)
(source)
On the other hand, in general terms, intensity of emission is proportional to the transition moment, $$\mathrm{TM} = \langle \Psi_i | \mu | \Psi_f \rangle$$ where $\Psi_i, \Psi_j$ are the initial and final molecular wavefunctions, and $\mu = q_e \sum \vec r_i$ is the dipole moment. This expression doesn't seem to be related to polarizability.
Then why would electronic transitions ($\Psi_i\to\Psi_f$, i.e. photoluminescence yield) depend on polarizability? In other words, why does Raman activity depend on polarizability?