Can a single particle be “heated” by radiation?
Not really, because heat is an emergent macroscopic property of an ensemble of particles. But since you put the word "heated" in quotes, we can allow a yes of sorts. Take a look at the Wikipedia temperature page, where we can see this picture:
 CCASA image by Greg L, see Wikipedia
CCASA image by Greg L, see Wikipedia
It's to do with the kinetic theory of gases. A hot gas is one where the molecules are moving fast. And radiation will heat this gas. It will makes the molecules move faster. The simplest version of this is Compton scattering, see Rod Nave's hyperphysics:
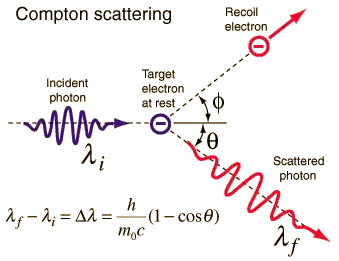
The single particle is "heated" by radiation because a fast electron is a "hot" electron. Google it.
From the point of view of statistical thermodynamics, a single particle doesn't have a phase (state of matter), nor temperature.
Yes, but thermodynamics isn't just statistical dynamics. Look at the name and its etymology. "It was first spelled in a hyphenated form as an adjective (thermo-dynamic) in 1849 and from 1854 to 1859 as the hyphenated noun thermo-dynamics to represent the science of heat and motive power". Motive power. Motion.
What would happen if heat is transported to this single particle via radiation?
Its energy is increased. But this is kinetic energy rather than rest-mass energy.
How is this related to the same thing occurring for a mass of particles?
If the particles are confined inside a box, their kinetic energy is effectively rest-mass energy. They aren't actually at rest, but the average position of the particles with respect to you doesn't change. The mass of the system increases because of the added energy. A box full of fast-moving particles is harder to accelerate than a box full of slow-moving particles.
 CCASA image by Greg L, see
CCASA image by Greg L, see 