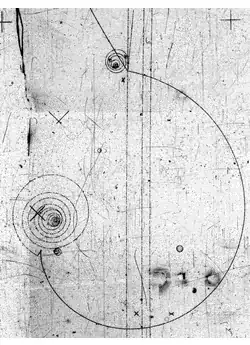
In classical physics we have always dealt with the motion of non-punctual objects as fluids and rigid bodies (let’s call them fields). The trajectory of those objects is clearly not a single line. However, by experience we notice that such fields have special symmetry points were all other points seem to move around. These points are known as the center of mass (or better, center of energy) and therefore, the trajectory of a field can be decomposed into two. One is the clear path of the center of mass and other is motion of every individual point around it. In quantum mechanics I always view particles as fields just as in classical mechanics. You can call these as probability density fields $\Psi(p)=|\psi(p)|^2$, but I prefer to call them THE “particle distribution”. Such a distribution governs the distribution of every other property of the particle.
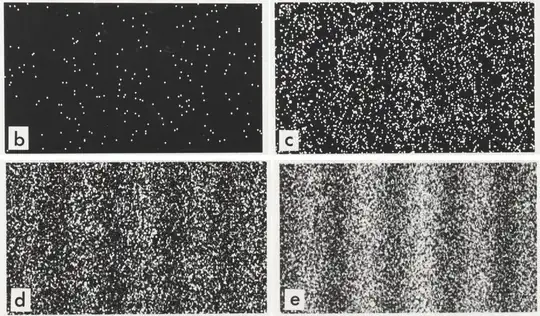 Single point interation pattern of particles. By Dr. Tonomura and Belsazar
Single point interation pattern of particles. By Dr. Tonomura and Belsazar
Now, if particles are volumetric, then why particle interaction with a screen builds up as single points like in the image above? Well, by the same reason one can empty an entire bathtub thru a single hole. In fact, we could drain an entire ocean from a small hole, if we could make that hole down to the center of earth and create some room there for it. Fluids have 2 characteristics that make them prone to do that: 1) If you remove some of the fluid in a particular point, disturbing they natural shape, it brings fluid from the vicinity to restore its original shape; 2) The bigger the gradient (removal) the faster it pours into that point.
Characteristic 2) is important because, it means that a fast passing particle will have a bigger change to pour all of its energy into a particular point before getting too far from it, if the value of its energy distribution is high enough at that point to allow for a big uptake. This means that the higher the value of the “fluid distribution” in a particular point, the higher the probability for the particle to strongly interact in that point, i.e. $\Psi(p)$ gives the probability density that a particle will (strongly) interact into a particular point.
If you view particles this way, many problems of quantum physics become trivial. Let me give you some examples:
- The double slit experiment: particle passed thru both holes with those causing a reshape of the particle from $\Psi_1$ to $\Psi_2$. For example, from spherical symmetry into peaks and valleys, causing the interaction pattern in the screen to change from a located small dot into an interference pattern;
- The uncertainty principle: You cannot find a particle in an infinitesimally small volume. The smaller the volume you integrate the “particle distribution”, the bigger the chunk of the particle you miss;
- The non-irradiation nature of standing electrons in an atom: Electrons (particles) can trade shape for an equivalent amount of energy (and momentum) just as you can do by reshaping a spring or a rubber band. They can trade “moving energy” (kinetic energy) into an equivalent “standing energy” (potential energy). For example, the electron cloud in the orbitals, are the electron themselves and they are not moving. If they move from one shape into another, this means that they will have to eliminate some momentum and energy equivalent to the difference of these quantities from the old to the new setup.
Nevertheless, there are 2 points that need to be clarified:
First, why particle seems to always interact in a single point, even when $\Psi$ have equally high values at multiple points? To answers this, we need to understand that in order to have a strong interaction, we need a sink (other particles, fields, etc). Due to the chaotic nature and instantaneous existence of those in a particular point the interaction happens for the first one that gathers all necessary things first. In other words, in a particle sinking contest, the winner takes it all.
Second and more importantly, what is the nature of that “particle distribution” I mention before? For that question I don’t have an answer. One needs to remind himself that $\psi$ is a spinor field. There is no equivalent to that in classical physics, so maybe it is just out of our intuition to imagine a quantity like that, but, for now, the probability wave nature of $\psi$ seems to work, and I don’t want to rock the boat.
In summary:
- Particles have defined trajectories that correspond to their “center of distribution’s” path, even though they may not strongly interact in that point, as it could be in a valley. This is a similar case to that of a hollow rigid sphere in classical physics;
- It seems that high energy free particles tend have their strong interaction probability concentrated around their “center of distribution” as I pointed out in this question, causing them to mostly behave as a particle with clear trajectories rather than a wave.