I've got asked by someone who just graduated school and is about to start studying physics, what exactly is an electron, if it is not "a small ball rotating around the core of an atom". I couldn't really break it down for him though, i guess he didn't understand my explanation. Do you have an explanation in "easy words" that is still physically correct?
4 Answers
Electron is a particle with mass and a certain probability of being found at a given distance around the nucleus of an atom at a certain time. It caries a negative charge which makes chemical reactions possible since chemical reactions are driven by the electrostatic forces between electrons and positively charged protons which reside in the nucleus of the atom along with neutrally charged neutrons. It also allows for the flow of electricity, since the flow of current is directly proportional to electron flow. However if current is flowing one way, then the electros are flowing the opposite way ie current is a measure of positive charge flow.
- 171
Here is a more complicated answer:
I am going to try my best, ok?
An electron is a negative elementary charge subatomic particle of an atom. It was once known as a beta particle, but it is now an electron. It takes electromagnetic, gravity, and weak interactions into play. In Coulombs, it's charge is approximately $-1.6 * 10^{-19} C$ and it's mass in kilograms is approximately $9.1*10^{-31} kg$. It is said that the electron has no substructure, meaning there is no subparticle known that makes up the electrons. It was first discovered by J. J. Thomson in 1897 in an experiment to observe the straightness of a cathode ray (I think, someone correct me on that if I'm wrong), who also theorized that these particles existed like "blueberries in a muffin", meaning they were almost implanted on the surface of an atom. With the discovery of the nucleus of an atom (from an experiment which fired alpha particles through a slit and found that their paths were changed drastically), Rutherford said that electrons orbited the nucleus much like planets do around the sun. However, this model failed to explain the stability of the atoms (as since the electrons have to counteract the pull of charge and gravitation with centrifugal force and electromagnetic pulses), and also failed to explain the ray spectrums that were emitted from the gases when light was shown through them instead of a continuous spectrum. In comes the Bohr Model which apparently solves all those problems and more. The Bohr model states the quantum condition, $2\pi rmv = nh$, and the frequency condition, $hf = E_f - E_i$, this also used the proposition from french scientist de Boglie that all moving particles take the properties of a wave ( wave particle duality ). However, the Bohr model failed to explain many other Spectrum phenomena, but Schrodinger used his equations to create wave functions which explained many of them, and Heisenberg using quantum mechanics set forth the Uncertainty Principle, which was used to create the model of today, which includes an electron cloud where an electron could be theorized to be there in that area. The electron again, orbits the nucleus based on it's energy levels at different radii from the nucleus. This radii can be quantitized and used to explain the absorption and emission ray spectrums caused by the atoms. Basically, electrons have the tendency to "jump" between energy level orbits in an atom. Typically, the number of electrons in a specific elements valence shell determines it's ability to conduct electricity or form chemical reactions with other elements. It's transition to an atom's conduction band also allows the specific element to flow currents. The transition of electrons from one atom to another also causes electric fields to form, and either it's moving orbit or half-spin allows for magnetic fields to form as well.
Phew! Someone please tell me if I got something wrong and if you can edit it, but show the edits, I want to learn from my mistakes.
- 277
Hmmm... I'll take a crack at it.
An electron is a negatively charged subatomic particle that orbits the nucleus of an atom, which contains a positively charged subatomic particle called a proton and a neutral subatomic particle called a neutron.
- 277
How to explain what an electron is to someone new to physics?
I think you can come up with an easy-reading explanation that's physically correct. There's plenty of clues in the literature if you're willing to play detective. For example see pair production. We quite literally make an electron (and a positron) out of light. And then when we annihilate the electron and the positron, we get the light back. So:
1) The electron is quite literally made from light.
Then there's things like electron diffraction. We can diffract electrons, just like we can diffract light. We can also diffract neutrons, and buckyballs. We can also refract electrons, see The Refractive Index in Electron Optics and the Principles of Dynamics. That's the paper that predicted what's now known as the Aharonov-Bohm effect. The wave nature of matter is not in doubt. So:
2) The electron is definitely a wave.
After that we have things like Goudsmit and the discovery of electron spin: "But don't you see what this implies? It means that there is a fourth degree of freedom for the electron. It means that the electron has a spin, that it rotates". On top of that there's the Einstein-de Haas effect which " demonstrates that spin angular momentum is indeed of the same nature as the angular momentum of rotating bodies as conceived in classical mechanics". So:
3) The electron is an electromagnetic wave going round and round.
And then of course we talk about Dirac's belt wherein "a Möbius strip is reminiscent of spin-1/2 particles in quantum mechanics, since such particles must be rotated through two complete rotations in order to be restored to their original state". We also talk about spinors, which we similarly depict, with a chirality. And then in atomic orbitals the electrons "do not orbit the nucleus in the sense of a planet orbiting the sun, but instead exist as standing waves". And of course when we're referring to atomic orbitals we talk of spherical harmonics. And there's also topological quantum field theory which is related to knot theory. So:
4) The electron is a 511keV electromagnetic wave going round and round in a knot-like stable spherical spin-½ spinor configuration, such that the field-variation propagating at c now looks like an all-round standing field. Standing wave, standing field. In its simplest guise within an atom we call it an S-orbital, which is depicted below:
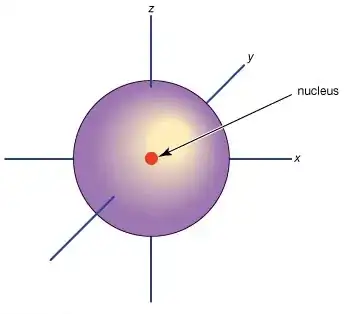 © Copyright 2010 Encyclopaedia Britannica
© Copyright 2010 Encyclopaedia Britannica
It's important to note that a standing wave is a wave which appears to be motionless, when actually it isn't. If you have a standing wave in a cavity, when you "open the box" the wave is off like a shot from a standing start, because it wasn't really standing. It's the same for electron-positron annihilation to gamma photons. And to visualize the electron wave motion, see the "spindle sphere torus" below, courtesy of Adrian Rossiter's torus animations.

- 11,381