What does it mean to have 'half' spin? I have looked on Wikipedia and a few youtube videos on spin but they don't explain what it means to have $1/2$ spin. I am 18 and only starting to learning about quantum mechanics not so long ago, so please keep the vocabulary to a minimal.
1 Answers
Quantum mechanics (QM; also known as quantum physics, or quantum theory) is a fundamental branch of physics which deals with physical phenomena at nanoscopic scales, where the action is on the order of the Planck constant
The Planck constant is a very small number, 6.6*10^-34 Joulesecond
Quantum mechanics was invented because the data showed that at these small dimensions measurable variables were often not continuous, but came in packets eventually called quanta. The necessity for this solution came from the photoelectric effect, the black body radiation, the discrete spectra of excited atoms, and it has been experimentally established that quantum mechanics is the underlying level of nature. For every measurable observable there corresponds a quantum mechanical operator which operating on the quantum mechanical state gives the probability of measuring the specific measurement. In the case of the operator corresponding to the angular momentum, the values are quantized. This theory developed because of the observation of quantization in orbital angular momentum in the solutions describing atoms.
It was then found experimentally that there existed an intrinsic angular momentum (named spin) characterizing particles like protons, neutrons, electrons which make up atoms and molecules. Spin 1/2 is the smallest quantum of angular momentum, conceptually in the same way that charge +/- 1/3 is the smallest quantum assignable to elementary particles..
The spin of the electrons is 1/2*h_bar, where
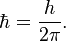
As elementary particles make up all matter, by algebra the only allowed values for spin are multiples of 1/2 and angular momentum multiples of 1 times h_bar. The smallness of the constant ensures that at macroscopic values angular momentum is to all intents and purposes continuous.
Thus the real answer is "because that is what we have observed to be the case in the microscopic interactions of particles".
- 236,935