Actually, in a sense, there does exist something which moves. It's not something which can be measured to move, but rather part of quantum mechanical description, evolution of which much resembles classical electron motion: the phase of wavefunction.
See this java demonstration of hydrogen atom orbital. If you select "Complex Orbitals (phys.)" and then choose a state with $m\ne0$, then you'll see how the colours "rotate". This shows that the phase of the wavefunction does rotate around the $z$ axis. In a sense, this can be said to create the orbital magnetic moment.
But please note, I'll repeat: this is not an observable rotation by itself! Moreover, the angular velocity of phase is actually not fixed - it can be shifted by arbitrary constant (provided it's the same for all the states). But it does show how the wavefunction evolves in time. If you try to measure anything, all you can get is the magnitude squared of the wavefunction (for a large enough number of identical experiments). Still, the phase does play a role in creation of superpositions of states and finally in allowing the probability densities change in time, while for stationary states the probability densities are constant in time, as you already know.
EDIT in response to comment:
First, the wavefunction is not real in the sense $\psi\not\in\mathbb R$. It's complex, while all the directly measurable quantities must be real.
Second, despite in eigenstate the atom is stationary, we can make linear combinations of eigenstates with the same direction of magnetic moment such, that the result will be a wave packet measurably rotating in a definite direction.
For a simpler example of translational motion instead of rotational, consider a plain wave. Here's how it looks (blue real part, purple imaginary, yellow square of magnitude):
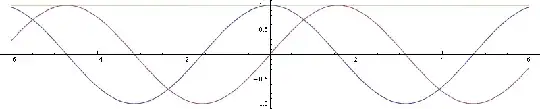
Now let's make up a Gaussian wave packet, like in this demonstration. It'll have the same peak state, but additionally some more states with higher and lower phase velocities (with smaller amplitudes). Here's its wave function (blue real part, purple imaginary):
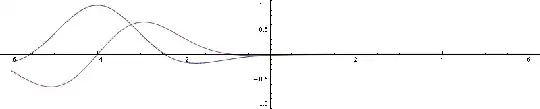
Now this packet has measurable motion. Here's its square of magnitude, which is the probability density by Born rule:
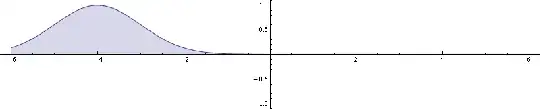
You see that for a particle eigenstate, which is a plane wave, you can't measure its motion. But OTOH, it's the limit of infinitely delocalized wave packet, and for a localized wave packet you can measure its motion (in probabilistic sense, of course).
The same is for rotational motion, it just has some more technical features, which aren't relevant for understanding here.


